The Latest Edition Of #PeriodicGraphics In C&EN Looks At Some Fruits And Vegetables Which We Might Not

The latest edition of #PeriodicGraphics in C&EN looks at some fruits and vegetables which we might not consider dangerous, but which can, in some cases, contain unwelcome natural toxins: https://ift.tt/3fNzwOE https://ift.tt/2K60CoM
More Posts from Amateurchemstudent and Others
Covalent Bonds: Sharing Is Caring!
Welcome to my second out of three posts on bonding - ionic, covalent and metallic. This post also covers the coordinate/ dative bond which I can’t remember if I’ve covered before. Only one more of this series left! Find the others here.
Covalent bonding involves one or more shared pairs of electrons between two atoms. These can be found in simple molecular elements and compounds like CO2 , macromolecular structures like diamond and molecular ions such as ammonium. Covalent bonds mostly occur between non-metals but sometimes metals can form covalent bonds.
Single covalent bonds share just one pair of electrons. Double covalent bonds share two. Triple covalent bonds share three.
Each atom usually provides one electron – unpaired in the orbital – in the bond. The number of unpaired electrons in an atom usually shows how many bonds it can make but sometimes atoms promote electrons to fit in more. Covalent bonds are represented with lines between the atoms – double and triple bonds represented with two and three lines respectively.
Dot and cross diagrams show the arrangement of electrons in covalent bonds. They use dots and crosses to demonstrate that the electrons come from different places and often only the outer shell is shown.

The simple explanation as to how atoms form covalent bonds is that one unpaired electron in the orbital of one atom overlaps with one in another atom. Sometimes atoms promote electrons in the same energy level to form more covalent bonds. For example, if an atom wants to make three covalent bonds but has a full 3s2 shell and a 3p1 shell, it can promote one of its 3s2 electrons so that an electron from the other atoms can fill the 3s shell and pair with the new 3p2 shell.
Sometimes promotion does not occur and that means different compounds can be made such as PCl3 or PCl5.

A lone pair of electrons is a pair of electrons from the same energy sub-level uninvolved in bonding. Sometimes these can form something called a coordinate bond, which contains a shared pair of electrons where both come from one atom. The lone pair of electrons is “donated” into the empty orbital of another atom to form a coordinate bond.

This is an example of a coordinate (sometimes called dative) bond between ammonia and a H+ ion which has an empty orbital. The lone pair on the ammonia overlaps with this H+ ion and donates its electrons. Both electrons come from the ammonia’s lone pair so it is a coordinate bond. This is demonstrated with an arrow. The diagram is missing an overall charge of + on the ammonium ion it produces. Coordinate bonds act the same as covalent bonds.
Once you have your covalent bonds, you need to know about covalent substances and their properties. There are two types of covalent substance: simple covalent (molecular) and macromolecular (giant covalent).
Molecular simply means that the formula for the compound or element describes exactly how many atoms are in one molecule, e.g. H2O. Molecular covalent crystalline substances usually exist as single molecules such as iodine or oxygen. They are usually gases or liquids at room temperature but can be low melting point solids.
Solid molecular covalent solids are crystalline so can be called molecular covalent crystals. Iodine and ice are examples of these. Iodine (shown below) has a regular arrangement which makes it a crystalline substance and water, as ice, has a crystalline structure as well.

The properties of these crystals are that they have low melting points, are very brittle due to the lack of strong bonds holding them together and also do not conduct electricity since no ions are present.
The other kind of covalent substance you need to know is macromolecular. This includes giant covalent structures such as diamond or graphite, which are allotropes of carbon. Non-metallic elements and compounds usually form these crystalline structures with a regular arrangement of atoms.
Allotropes are different forms of the same element in the same physical state.
Diamond is the hardest naturally occurring substance on earth therefore is good for cutting glass and drilling and mining. It has a high melting point due to the many covalent bonds which require a lot of energy to break. Each carbon has four of these bonds joining it to four others in a tetrahedral arrangement with a bond angle of 109.5 degrees and it does not conduct electricity or heat because there are no ions free to move.
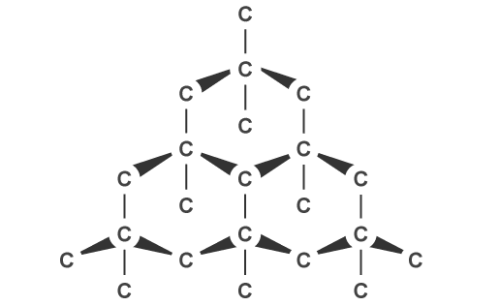
Graphite, on the other hand, can conduct electricity. This is because it has delocalised electrons between the layers which move and carry charge. Carbon atoms within the structure are only bonded to three others in a hexagonal arrangement with a bond angle of 120 degrees. Since only three of carbon’s unpaired electrons are used in bonding, the fourth becomes delocalised and moves between the layers of graphite causing weak attractions, explaining why it can conduct electricity.

Graphite’s layered structure and the weak forces of attractions between it make it a good lubricant and ideal for pencil lead because the layers can slide over each other. The attractions can be broken easily but the covalent bonds within the layers give graphite a high melting point due to the amount of energy needed to break them.
SUMMARY
Covalent bonding involves one or more shared pairs of electrons between two atoms. Covalent bonds mostly occur between non-metals but sometimes metals can form covalent bonds.
Single covalent bonds share just one pair of electrons. Double covalent bonds share two. Triple covalent bonds share three.
Each atom usually provides one electron – unpaired in the orbital – in the bond. The number of unpaired electrons in an atom usually shows how many bonds it can make but sometimes atoms promote electrons to fit in more. Covalent bonds are represented with lines between the atoms.
Dot and cross diagrams use dots and crosses to demonstrate that the electrons come from different places and often only the outer shell is shown.
The simple explanation as to how atoms form covalent bonds is that one unpaired electron in the orbital of one atom overlaps with one in another atom. Sometimes atoms promote electrons in the same energy level to form more covalent bonds.
Sometimes promotion does not occur and that means different compounds can be made such as PCl3 or PCl5.
A lone pair of electrons is a pair of electrons from the same energy sub-level uninvolved in bonding. Sometimes these can form something called a coordinate bond, which contains a shared pair of electrons where both come from one atom. The lone pair of electrons is “donated” into the empty orbital of another atom to form a coordinate bond.
The formation of ammonium is an example of this.
There are two types of covalent substance: simple covalent (molecular) and macromolecular (giant covalent).
Molecular simply means that the formula for the compound or element describes exactly how many atoms are in one molecule, e.g. H2O. Molecular covalent crystalline substances usually exist as single molecules such as iodine or oxygen. They are usually gases or liquids at room temperature but can be low melting point solids.
Solid molecular covalent solids are crystalline so can be called molecular covalent crystals. Iodine and ice are examples of these.
The properties of these crystals are that they have low melting points, are very brittle due to the lack of strong bonds holding them together and also do not conduct electricity since no ions are present.
Giant covalent structures such as diamond or graphite are allotropes of carbon. Allotropes are different forms of the same element in the same physical state.
Diamond has a high melting point due to the many covalent bonds which require a lot of energy to break. Each carbon has four of these bonds joining it to four others in a tetrahedral arrangement with a bond angle of 109.5 degrees and it does not conduct electricity or heat because there are no ions free to move.
Graphite can conduct electricity. This is because it has delocalised electrons between the layers which move and carry charge. Carbon atoms within the structure are only bonded to three others in a hexagonal arrangement with a bond angle of 120 degrees. Since only three of carbon’s unpaired electrons are used in bonding, the fourth becomes delocalised and moves between the layers of graphite causing weak attractions, explaining why it can conduct electricity.
Graphite’s layered structure and the weak forces of attractions between it make it a good lubricant and ideal for pencil lead because the layers can slide over each other. The attractions can be broken easily but the covalent bonds within the layers give graphite a high melting point due to the amount of energy needed to break them.
Happy studying!
It’s World Sleep Day
Log off.
Go back to bed.
i just learned from animal crossing that pondskaters stay on top of the water by secreting an oil from their feet
that seems kinda obvious in hindsight. i always figured they were just, like, light enough to not break surface tension
Alkanes: Saturated Hydrocarbons
So you want to be an organic chemist? Well, learning about hydrocarbons such as alkanes is a good place to start…
Alkanes are a homologous series of hydrocarbons, meaning that each of the series differs by -CH2 and that the compounds contain carbon and hydrogen atoms only. Carbon atoms in alkanes have four bonds which is the maximum a carbon atom can have - this is why the molecule is described to be saturated. Saturated hydrocarbons have only single bonds between the carbon atoms.
The general formula of an alkane is CnH2n+2 where n is the number of carbons. For example, if n = 3, the hydrocarbon formula would be C3H8 or propane. Naming alkanes comes from the number of carbons in the chain structure.
Here are the first three alkanes. Each one differs by -CH2.

Shorter chain alkanes are gases at room temperature, medium ones are liquids and the longer chain alkanes are waxy solids.
Alkanes have these physical properties:
1. They are non-polar due to the tiny difference in electronegativity between the carbon and hydrogen atoms.
2. Only Van der Waals intermolecular forces exist between alkane molecules. The strength of these increase as relative molecular mass increases therefore so does the melting/boiling point.
3. Branched chain alkanes have lower melting and boiling points than straight chain isomers with the same number of carbons. Since atoms are further apart due to a smaller surface area in contact with each other, the strength of the VDWs is decreased.
4. Alkanes are insoluble in water but can dissolve in non-polar liquids like hexane and cyclopentane. Mixtures are separated by fractional distillation or a separating funnel.
The fractional distillation of crude oil, cracking and the combustion equations of the alkanes will be in the next post.
SUMMARY
Alkanes are a homologous series of hydrocarbons. Carbon atoms in alkanes have four bonds which is the maximum a carbon atom can have - this is why the molecule is described to be saturated. Saturated hydrocarbons have only single bonds between the carbon atoms.
The general formula of an alkane is CnH2n+2 where n is the number of carbons.
Shorter chain alkanes are gases at room temperature, medium ones are liquids and the longer chain alkanes are waxy solids.
They are non-polar.
Only Van der Waals intermolecular forces exist between alkane molecules. The strength of these increase as relative molecular mass increases therefore so does the melting/boiling point.
Branched chain alkanes have lower melting and boiling points than straight chain isomers with the same number of carbons.
Alkanes are insoluble in water but can dissolve in non-polar liquids like hexane. Mixtures are separated by fractional distillation or a separating funnel.
🌻 little habits/things to do more of 🌻
dailies
make your bed. (no, really.)
set your top 3 to-dos for the day.
do your top 3 to-dos for the day. (heh)
stretch.
unpack your bag when you get home.
prepare your things for the next day before sleeping.
skincare. (your basic cleanse and moisturize)
sweep the floor of your bedroom.
talk to your plants. (if you have plants)
update your financial report/expense tracker.
take a good photo.
meditate.
hug at least three people. (seriously.)
weeklies
polish your school shoes.
mop your bedroom floor.
dare i say, laundry. (don’t put it off!)
exfoliate.
take a leisure walk.
review your past week and plan your next week accordingly. (a part of your routine may not be working–try something new)
make a piece of art. (a sketch, a collage, a quote in pretty lettering, a god-awful poem..)
sanitize your gadgets. (whip out the wet tissue and wipe away at your phone, your laptop, your mouse, your earphones–just don’t forget to IMMEDIATELY follow that up with a dry cloth to prevent fogging and short circuits)
watch a TED Talk.
make a new playlist.
monthlies
wash your bag.
wash your shoes.
change the sheets of your bed and your pillows.
clip your nails. (honestly)
wax/shave. (if you want. i just really like how fresh my skin feels after i torture it with razors and wax strips)
wipe your shelves/the tops of your furniture clean. (try to avoid dusting. it just scatters the dirt everywhere. use a damp cloth instead)
see if there’s anything in your storage that you don’t need/want anymore and give stuff away or sell them.
review your month and plan the next one accordingly. (just like your weeks. remember to refer to your Life Goal/Year’s Goals page)
finish reading at least one book. (and review it!)
discover new songs.
- 🍂
Breaking Down Alkanes - isn’t it cracking?
Unfortunately, if you’re sitting your A Level chemistry exam, you need to know a little more than the basic properties of alkanes outlined in my last post. Luckily though, this post takes you through fractional distillation and the two types of cracking - isn’t that convenient?
Crude oil contains carbon compounds formed by the effects of pressure and high temperature on plant and animal remnants. It is viscious, black and found in rocks beneath the earth’s surface. It is a mixture of mainly alkane hydrocarbons which are separated by a process called fractional distillation. Crude oil is essential because it is burned as a fuel and each fraction has different properties e.g. diesel, petrol, jet fuel.
Fractional distillation is the continual evaporation and condensation of a mixture which causes fractions to split due to a difference in boiling point. It is important to note that fractional distillation does not separate crude oil into pure compounds but rather less complex mixtures. Fractions are groups of compounds that have similar boiling points and are removed at the same level of a fractionating column.
The first step in this process is to heat crude oil in a furnace until some changes state from a liquid to a vapour. This mixture goes up a fractionating tower or column which is hotter at the bottom than the top and reaches a layer which is cool enough to condense and be collected. Shorter chain molecules are collected at the top where it is cooler since they have lower boiling points.

As you go down the fractionating column, bear in mind that: the column temperature increases, the boiling point increases, the number of carbon atoms increases and the strength of the Van der Waals’ between molecules increases.
Different fractions have different usefulnesses and often, it is the fractions with lower boiling points and shorter chains which are much more purposeful. Therefore there needs to be a process to getting shorter chains because they are the least abundant in crude oil samples. To meet demand, long chain molecules that are less useful are broken down into shorter chain molecules. This is done by cracking.
Cracking is a process where long chain hydrocarbon molecules are broken down into shorter chain molecules which are in high demand. This can be done one of two ways - thermal or catalytic.
Thermal cracking involves heating long chain alkanes to high temperatures - usually between 1000 - 1200K. It also uses high pressures up to 70atm and takes just one second. It only needs a second because the conditions could decompose the molecule completely to produce carbon and hydrogen instead. The conditions produce shorter chain alkanes and mostly alkenes.
A typical equation for this:

Decane -> octane + ethene
C10H22 -> C8H18 + C2H4
Catalytic cracking also breaks down long alkanes by heat under pressure using the presence of a zeolite catalyst. Temperature used is approx. 800-1000K and the pressure is often between 1-2 atm. Zeolite is an acidic mineral with a honeycomb structure, made from aluminium oxide and silicion dioxide. The honeycomb structure gives the catalyst a larger surface area which increases ROR. Factories which catalytically crack are often operated continuously for around 3 years at a time and produce branched alkanes, cycloalkanes and aromatic compounds.
You need to be able to compare the conditions of catalytic and thermal cracking for the A Level exam. Know that thermal cracking has a high temperature and pressure, a short duration, no catalyst and produces a high percentage of alkenes and some short chain alkanes. Catalytic uses a catalyst, a high temperature, a low pressure and produces aromatic hydrocarbons and motor fuels.
SUMMARY
Crude oil contains carbon compounds formed by the effects of pressure and high temperature on plant and animal remnants. I It is a mixture of mainly alkane hydrocarbons which are separated by a process called fractional distillation.
Fractional distillation is the continual evaporation and condensation of a mixture which causes fractions to split due to a difference in boiling point.
It is important to note that fractional distillation does not separate crude oil into pure compounds but rather less complex mixtures.
Fractions are groups of compounds that have similar boiling points and are removed at the same level of a fractionating column.
The first step in this process is to heat crude oil in a furnace until some changes state from a liquid to a vapour. This mixture goes up a fractionating tower or column which is hotter at the bottom than the top and reaches a layer which is cool enough to condense and be collected. Shorter chain molecules are collected at the top where it is cooler since they have lower boiling points.
As you go down the fractionating column, bear in mind that: the column temperature increases, the boiling point increases, the number of carbon atoms increases and the strength of the Van der Waals’ between molecules increases.
Fractions with lower boiling points and shorter chains are much more purposeful but are the least abundant in crude oil samples. To meet demand, long chain molecules that are less useful are broken down into shorter chain molecules.
Cracking is a process where long chain hydrocarbon molecules are broken down into shorter chain molecules which are in high demand.
Thermal cracking involves heating long chain alkanes to high temperatures - usually between 1000 - 1200K. It also uses high pressures up to 70atm and takes just one second. It only needs a second because the conditions could decompose the molecule completely to produce carbon and hydrogen instead. The conditions produce shorter chain alkanes and mostly alkenes.
Catalytic cracking also breaks down long alkanes by heat under pressure using the presence of a zeolite catalyst. Temperature used is approx. 800-1000K and the pressure is often between 1-2 atm. Zeolite is an acidic mineral with a honeycomb structure, made from aluminium oxide and silicion dioxide. The honeycomb structure gives the catalyst a larger surface area which increases ROR.
You need to be able to compare the conditions of catalytic and thermal cracking for the A Level exam. Know that thermal cracking has a high temperature and pressure, a short duration, no catalyst and produces a high percentage of alkenes and some short chain alkanes. Catalytic uses a catalyst, a high temperature, a low pressure and produces aromatic hydrocarbons and motor fuels.
Happy studying!
Universities are like "we can't accept everyone based on accepted grades because we gave too many offers out." They give out too many offers because they're horrified at the thought that they might end up with too many empty places on courses, so they oversubscribe so they can get those sweet sweet tuition fees.
Just in case anyone thought here was a thing that Tony Blair had no hand in, for once.
Shapes of Molecules
his post is more information than trying to explain something - the truth is, you just need to learn shapes of molecules like you do with anything. I’ve got a physical chemistry mock tomorrow that I’m dreading since I’ve done zero revision. The fact that I run a study blog yet don’t revise myself is odd, but what else can I do? Oh, wait … revise. So here it is, my last minute revision for myself and you too - I present, shapes of molecules!
VSEPR stands for valence shell electron pair repulsion theory. If you’ve ever seen a moly-mod or a diagram of a molecule in 3D space, you may wonder how they decided it was that shape. Well, VSEPR answers all.
The theory essentially states that electron pairs are arranged to minimise repulsions between themselves - which makes sense, since electrons carry the same charge and therefore try to repel each other. Of course, there are different types of electron pairs, lone and bonding. The strongest repulsions happen between lone pair - lone pair followed by lone pair - bonding pair and finally, bonding pair - bonding pair have the least repulsion.
Since the repulsion governs the shape of the molecule, to work out a molecule’s shape you must look at dot and cross diagrams or electron configurations to see how a molecule is bonded. There are many methods to do this, but the bottom line is that you must work out how many bonding pairs of electrons and how many lone pairs are involved.
The easiest shape to learn is linear. This has two bonding pairs and no lone pairs at an angle of 180 degrees, since that is the furthest the two can get away from each other. Examples of linear molecules include carbon dioxide and beryllium chloride.


Next up is trigonal planar. This has three bonding pairs and no lone pairs, each at the angle of 120 degrees. Trigonal means three and planar means on one plane, this should help you in identifying the molecules since after a fourth pair of electrons, the shape becomes 3D. Examples of trigonal planar molecules include boron trifluoride and sulfur trioxide.


What if you were to have two bonding pairs and two lone pairs? Well, then you’d have a bent molecule. Water is a good example of a bent molecule. Since it has two lone pairs that repel the other two bonding pairs more than they repel each other, the bond angle is 104.5. I’d be careful though, as in many textbooks it shows a bent molecule to have one lone pair and a different bond angle.

Another variation of the bent molecule I’ve seen is the one with two bonding pairs and one lone pair. It is deemed as bent with a bond angle of 109 or sometimes less than 120 degrees.

Tetrahedral molecules have four bonding pairs and no lone pairs. The bond angle is 109.5 degrees. Examples of this include the ammonium ion, methane and the phosphate ion. A good thing to note here is how these molecules are drawn. To demonstrate the 3D shape, where the molecule moves onto a plane, it is represented with a dashed line and triangular line along with a regular straight line.


Trigonal pyramidal, sometimes just called pyramidal, is where there are three bonding pairs and a lone pair. Bond angles are roughly 107 degrees due to the repulsion from the lone pairs. An example of a trigonal pyramidal molecule is ammonia, which has a lone pair on the nitrogen.

Having five bonding pairs gives a trigonal bipyramidal structure. I guess the three bonding pairs on the trigonal plane accounts for that part of the name, where the rest comes from the position of the remaining two. These molecules have no lone pairs and have a bond angle of 90 degrees between the vertical elements and 120 degrees around the plane. Diagrams below are much clearer than my description! Examples of this include phosphorus pentachloride.


Six bonding pairs is an octahedral structure. I know this is confusing because octahedral should mean 8 but it’s one of those things we get over, like the fact sulfur isn’t spelt with a ph anymore. It’s actually to do with connecting the planes to form an octahedral shape.There are no lone pairs and each bond angle is a nice 90 degrees. Common examples include sulfur hexafluoride.


Square planar shapes occur when there are six bonding pairs and two lone pairs. All bond angles are 90 degrees! They take up this shape to minimise repulsions between electrons - examples include xenon tetrafluoride.

The final one to know is T-shape. This has three bonding pairs and two lone pairs. These molecules have bond angles of (less than) 90 degrees, usually a halogen trifluoride like chlorine trifluoride.

There are plenty more variations and things you could know about molecular geometry, but the truth is, there won’t be an extensive section on it. It’s a small part of a big topic!
I’m not going to do a summary today since I’d just be repeating the same information (I tried to keep it concise for you guys) so instead I’ll just leave you with,
Happy studying!
Halogenoalkanes
Halogenoalkanes are a homologous series of saturated carbon compounds that contain one or more halogen atoms. They are used as refrigerants, solvents, flame retardants, anaesthetics and pharmaceuticals but their use has been restricted in recent years due to their link to pollution and the destruction of the ozone layer.
They contain the functional group C-X where X represents a halogen atom, F,Cl, Br or I. The general formula of the series is CnH2n+1X.
The C-X bond is polar because the halogen atom is more electronegative than the C atom. The electronegativity decreases as you go down group 7 therefore the bond becomes less polar. Flourine has a 4.0 EN whereas iodine has a 2.5 EN meaning it is almost non-polar.
The two types of intermolecular forces between halogenoalkane molecules are Van Der Waals and permanent dipole-dipole interactions. As the carbon chain length increases, the intermolecular forces (due to VDWs) increase as the relative atomic mass increases due to more electrons creating induced dipoles. Therefore the boiling point of the halogenoalkanes increases since more forces must be broken.
Branched chains have lower boiling points than chains of the same length and halogen because the VDWs are working across a greater distance and are therefore weaker.
When the carbon chain length is kept the same, but the halogen atom is changed, despite the effect of the changing polar bond on the permanent dipole-dipole interactions, the changing VDWs have a greater effect on the boiling point. Therefore as RAM increases, the boiling point increases meaning an iodoalkane has a greater boiling point than a bromoalkane if they have the same carbon chain length.
Halogenoalkanes are insoluble or only slightly soluable in water despite their polar nature. They are soluble in organic solvents such as ethanol and can be used as dry cleaning agents because they can mix with other hydrocarbons.
Summary
Halogenoalkanes are saturated carbon compounds with one or more halogen atoms. Their general formula is CnH2n+1X, where X is a halogen. Their functional group is therefore C-X.
They are used as refrigerants, solvents, pharmaceuticals and anaesthetics but have been restricted due to their link to the depletion of the ozone layer.
C-X bonds are polar due to the halogen being more electronegative than the carbon. The polarity of the bond decreases down group 7.
Van der Waals and permanent dipole-dipole interactions are the intermolecular forces in halogenoalkanes.
When carbon chain length increases, boiling points increase due to RAM increasing and the number of Van Der Waals increasing too.
In branched halogenoalkanes, Van Der Waals are working across a greater distance therefore attraction is weaker and boiling points are lower than an identical unbranched chain.
When the halogen is changed, the boiling point increases down the group due to the effect of a greater RAM - more VDWs mean more intermolecular forces to break.
Halogenoalkanes are insoluble in water but soluble in organic solvents like ethanol.
Bonus: free radical substitution reactions in the ozone layer
Ozone, O3, is an allotrope of oxygen that is usually found in the stratosphere above the surface of the Earth. The ozone layer prevents harmful rays of ultraviolet light from reaching the Earth by enhancing the absorption of UV light by nitrogen and oxygen. UV light causes sunburn, cataracts and skin cancer but is also essential in vitamin D production. Scientists have observed a depletion in the ozone layer protecting us and have linked it to photochemical chain reactions by halogen free radicals, sourced from halogenoalkanes which were used a solvents, propellants and refrigerants at the time.
CFCs cause the greatest destruction due to their chlorine free radicals. CFCs – chloroflouroalkanes – were once valued for their lack of toxicity and their non-flammability. This stability means that they do not degrade and instead diffuse into the stratosphere where UV light breaks down the C-Cl bond and produces chlorine free radicals.
RCF2Cl UV light —> RCF2● + Cl●
Chlorine free radicals then react with ozone, decomposing it to form oxygen.
Cl● + O3 —> ClO● + O2
Chlorine radical is then reformed by reacting with more ozone molecules.
ClO● + O3 —-> 2O2 + Cl●
It is estimated that one chlorine free radical can decompose 100 000 molecules of ozone. The overall equation is:
2O3 —-> 3O2
200 countries pledged to phase of the production of ozone depleting agents in Montreal, leading to a search for alternatives. Chemists have developed and synthesised alternative chlorine-free compounds that do not deplete the ozone layer such as hydroflurocarbons (HFCs) like trifluromethane, CHF3.
SUMMARY
Ozone, found in the stratosphere, protects us from harmful UV light which can cause cataracts, skin cancer and sunburn.
Ozone depletion has been linked to the use of halogenoalkanes due to their halogen free radicals.
CFCs were good chemicals to use because they have low toxicity and were non-flammable. The fact they don’t degrade means they diffuse into the stratosphere.
Chlorine free radicals are made when CFCs are broken down by UV light.
These go on to react with ozone to produce oxygen.
Chlorine free radicals are then reformed by reacting with more ozone.
It is a chain reaction that can deplete over 100 000 molecules of ozone.
There is a 200 country ban on their use and scientists have developed alternatives like hydrofluorocarbons to replace them
Happy studying!
-
 tajgernewdemon liked this · 8 months ago
tajgernewdemon liked this · 8 months ago -
 pleasurehunter2000 liked this · 4 years ago
pleasurehunter2000 liked this · 4 years ago -
 eternallyyours666 liked this · 4 years ago
eternallyyours666 liked this · 4 years ago -
 amateurchemstudent reblogged this · 4 years ago
amateurchemstudent reblogged this · 4 years ago -
 amateurchemstudent liked this · 4 years ago
amateurchemstudent liked this · 4 years ago -
 anactualamphibian reblogged this · 4 years ago
anactualamphibian reblogged this · 4 years ago -
 masternyaeon liked this · 4 years ago
masternyaeon liked this · 4 years ago -
 ohsunlight liked this · 4 years ago
ohsunlight liked this · 4 years ago -
 zacolyn reblogged this · 4 years ago
zacolyn reblogged this · 4 years ago -
 selfish-giant reblogged this · 4 years ago
selfish-giant reblogged this · 4 years ago -
 pharlapcartoonist liked this · 4 years ago
pharlapcartoonist liked this · 4 years ago -
 mpbte liked this · 4 years ago
mpbte liked this · 4 years ago -
 midgardgurl liked this · 4 years ago
midgardgurl liked this · 4 years ago -
 idol-kindergarden reblogged this · 4 years ago
idol-kindergarden reblogged this · 4 years ago -
 bug-catcher-jecht liked this · 4 years ago
bug-catcher-jecht liked this · 4 years ago -
 fat-ricky-sweetness liked this · 4 years ago
fat-ricky-sweetness liked this · 4 years ago -
 femtopulsed liked this · 4 years ago
femtopulsed liked this · 4 years ago -
 joeygrump reblogged this · 4 years ago
joeygrump reblogged this · 4 years ago -
 weeweewhirlwind liked this · 4 years ago
weeweewhirlwind liked this · 4 years ago -
 syntheticperson liked this · 4 years ago
syntheticperson liked this · 4 years ago -
 vineivy liked this · 4 years ago
vineivy liked this · 4 years ago -
 symbiotic-science reblogged this · 4 years ago
symbiotic-science reblogged this · 4 years ago -
 newyorkindiancouncil reblogged this · 4 years ago
newyorkindiancouncil reblogged this · 4 years ago -
 newyorkindiancouncil liked this · 4 years ago
newyorkindiancouncil liked this · 4 years ago -
 marcellessantos liked this · 4 years ago
marcellessantos liked this · 4 years ago -
 mauro-2002 liked this · 4 years ago
mauro-2002 liked this · 4 years ago -
 penpalmin reblogged this · 4 years ago
penpalmin reblogged this · 4 years ago -
 kitsuneukiuki liked this · 4 years ago
kitsuneukiuki liked this · 4 years ago -
 sparklemiranda liked this · 4 years ago
sparklemiranda liked this · 4 years ago -
 decathectx liked this · 4 years ago
decathectx liked this · 4 years ago -
 carola4u reblogged this · 4 years ago
carola4u reblogged this · 4 years ago -
 love029world liked this · 4 years ago
love029world liked this · 4 years ago -
 cabult reblogged this · 4 years ago
cabult reblogged this · 4 years ago -
 ok321letsgo liked this · 4 years ago
ok321letsgo liked this · 4 years ago -
 the-fisher-queen reblogged this · 4 years ago
the-fisher-queen reblogged this · 4 years ago -
 dolphingodd reblogged this · 4 years ago
dolphingodd reblogged this · 4 years ago -
 dolphingodd liked this · 4 years ago
dolphingodd liked this · 4 years ago
