Universities Are Like "we Can't Accept Everyone Based On Accepted Grades Because We Gave Too Many Offers
Universities are like "we can't accept everyone based on accepted grades because we gave too many offers out." They give out too many offers because they're horrified at the thought that they might end up with too many empty places on courses, so they oversubscribe so they can get those sweet sweet tuition fees.
Just in case anyone thought here was a thing that Tony Blair had no hand in, for once.
More Posts from Amateurchemstudent and Others
Covalent Bonds: Sharing Is Caring!
Welcome to my second out of three posts on bonding - ionic, covalent and metallic. This post also covers the coordinate/ dative bond which I can’t remember if I’ve covered before. Only one more of this series left! Find the others here.
Covalent bonding involves one or more shared pairs of electrons between two atoms. These can be found in simple molecular elements and compounds like CO2 , macromolecular structures like diamond and molecular ions such as ammonium. Covalent bonds mostly occur between non-metals but sometimes metals can form covalent bonds.
Single covalent bonds share just one pair of electrons. Double covalent bonds share two. Triple covalent bonds share three.
Each atom usually provides one electron – unpaired in the orbital – in the bond. The number of unpaired electrons in an atom usually shows how many bonds it can make but sometimes atoms promote electrons to fit in more. Covalent bonds are represented with lines between the atoms – double and triple bonds represented with two and three lines respectively.
Dot and cross diagrams show the arrangement of electrons in covalent bonds. They use dots and crosses to demonstrate that the electrons come from different places and often only the outer shell is shown.

The simple explanation as to how atoms form covalent bonds is that one unpaired electron in the orbital of one atom overlaps with one in another atom. Sometimes atoms promote electrons in the same energy level to form more covalent bonds. For example, if an atom wants to make three covalent bonds but has a full 3s2 shell and a 3p1 shell, it can promote one of its 3s2 electrons so that an electron from the other atoms can fill the 3s shell and pair with the new 3p2 shell.
Sometimes promotion does not occur and that means different compounds can be made such as PCl3 or PCl5.

A lone pair of electrons is a pair of electrons from the same energy sub-level uninvolved in bonding. Sometimes these can form something called a coordinate bond, which contains a shared pair of electrons where both come from one atom. The lone pair of electrons is “donated” into the empty orbital of another atom to form a coordinate bond.

This is an example of a coordinate (sometimes called dative) bond between ammonia and a H+ ion which has an empty orbital. The lone pair on the ammonia overlaps with this H+ ion and donates its electrons. Both electrons come from the ammonia’s lone pair so it is a coordinate bond. This is demonstrated with an arrow. The diagram is missing an overall charge of + on the ammonium ion it produces. Coordinate bonds act the same as covalent bonds.
Once you have your covalent bonds, you need to know about covalent substances and their properties. There are two types of covalent substance: simple covalent (molecular) and macromolecular (giant covalent).
Molecular simply means that the formula for the compound or element describes exactly how many atoms are in one molecule, e.g. H2O. Molecular covalent crystalline substances usually exist as single molecules such as iodine or oxygen. They are usually gases or liquids at room temperature but can be low melting point solids.
Solid molecular covalent solids are crystalline so can be called molecular covalent crystals. Iodine and ice are examples of these. Iodine (shown below) has a regular arrangement which makes it a crystalline substance and water, as ice, has a crystalline structure as well.

The properties of these crystals are that they have low melting points, are very brittle due to the lack of strong bonds holding them together and also do not conduct electricity since no ions are present.
The other kind of covalent substance you need to know is macromolecular. This includes giant covalent structures such as diamond or graphite, which are allotropes of carbon. Non-metallic elements and compounds usually form these crystalline structures with a regular arrangement of atoms.
Allotropes are different forms of the same element in the same physical state.
Diamond is the hardest naturally occurring substance on earth therefore is good for cutting glass and drilling and mining. It has a high melting point due to the many covalent bonds which require a lot of energy to break. Each carbon has four of these bonds joining it to four others in a tetrahedral arrangement with a bond angle of 109.5 degrees and it does not conduct electricity or heat because there are no ions free to move.
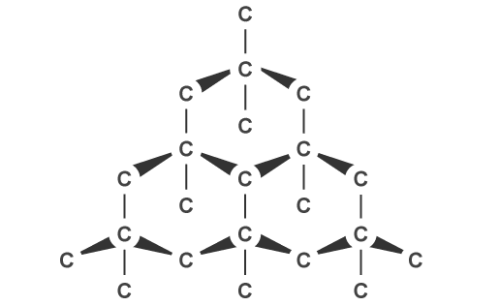
Graphite, on the other hand, can conduct electricity. This is because it has delocalised electrons between the layers which move and carry charge. Carbon atoms within the structure are only bonded to three others in a hexagonal arrangement with a bond angle of 120 degrees. Since only three of carbon’s unpaired electrons are used in bonding, the fourth becomes delocalised and moves between the layers of graphite causing weak attractions, explaining why it can conduct electricity.

Graphite’s layered structure and the weak forces of attractions between it make it a good lubricant and ideal for pencil lead because the layers can slide over each other. The attractions can be broken easily but the covalent bonds within the layers give graphite a high melting point due to the amount of energy needed to break them.
SUMMARY
Covalent bonding involves one or more shared pairs of electrons between two atoms. Covalent bonds mostly occur between non-metals but sometimes metals can form covalent bonds.
Single covalent bonds share just one pair of electrons. Double covalent bonds share two. Triple covalent bonds share three.
Each atom usually provides one electron – unpaired in the orbital – in the bond. The number of unpaired electrons in an atom usually shows how many bonds it can make but sometimes atoms promote electrons to fit in more. Covalent bonds are represented with lines between the atoms.
Dot and cross diagrams use dots and crosses to demonstrate that the electrons come from different places and often only the outer shell is shown.
The simple explanation as to how atoms form covalent bonds is that one unpaired electron in the orbital of one atom overlaps with one in another atom. Sometimes atoms promote electrons in the same energy level to form more covalent bonds.
Sometimes promotion does not occur and that means different compounds can be made such as PCl3 or PCl5.
A lone pair of electrons is a pair of electrons from the same energy sub-level uninvolved in bonding. Sometimes these can form something called a coordinate bond, which contains a shared pair of electrons where both come from one atom. The lone pair of electrons is “donated” into the empty orbital of another atom to form a coordinate bond.
The formation of ammonium is an example of this.
There are two types of covalent substance: simple covalent (molecular) and macromolecular (giant covalent).
Molecular simply means that the formula for the compound or element describes exactly how many atoms are in one molecule, e.g. H2O. Molecular covalent crystalline substances usually exist as single molecules such as iodine or oxygen. They are usually gases or liquids at room temperature but can be low melting point solids.
Solid molecular covalent solids are crystalline so can be called molecular covalent crystals. Iodine and ice are examples of these.
The properties of these crystals are that they have low melting points, are very brittle due to the lack of strong bonds holding them together and also do not conduct electricity since no ions are present.
Giant covalent structures such as diamond or graphite are allotropes of carbon. Allotropes are different forms of the same element in the same physical state.
Diamond has a high melting point due to the many covalent bonds which require a lot of energy to break. Each carbon has four of these bonds joining it to four others in a tetrahedral arrangement with a bond angle of 109.5 degrees and it does not conduct electricity or heat because there are no ions free to move.
Graphite can conduct electricity. This is because it has delocalised electrons between the layers which move and carry charge. Carbon atoms within the structure are only bonded to three others in a hexagonal arrangement with a bond angle of 120 degrees. Since only three of carbon’s unpaired electrons are used in bonding, the fourth becomes delocalised and moves between the layers of graphite causing weak attractions, explaining why it can conduct electricity.
Graphite’s layered structure and the weak forces of attractions between it make it a good lubricant and ideal for pencil lead because the layers can slide over each other. The attractions can be broken easily but the covalent bonds within the layers give graphite a high melting point due to the amount of energy needed to break them.
Happy studying!
What comes to mind when you think of alcohol? Probably alcoholic drinks like beer or wine. But in organic chemistry alcohols are an important and versatile family of compounds. In this episode of Crash Course Organic Chemistry, we’ll use alcohols as a starting point to get other types of compounds like ethers, epoxides, and more!
Biochemistry
Update: Pictures are working!
Atoms
There are a few basic chemistry concepts that are essential to understand. For starters, understanding what an atom is and its basic properties.
Atoms are the building block of all matter. They have a positive nucleus, with positive protons, and neutral neutrons. In a large area surrounding the nucleus, is the electron cloud, made of negatively charged electrons.
An atom in its elemental state is always neutral.
When an element has a charge, it is because it has an unequal number of protons an electrons, making it an ion. Sometimes an element’s nucleus has an unequal number of neutrons and protons, making it an isotope. Carbon-14, for example, has 8 neutrons, instead of the 6 that Carbon-12 has. Carbon-14 is also a radioisotope, meaning it emits particles and decays at a rate called a half-life, making it useful for fossil dating. Along with that, radioactive carbon can be used as a tracer. This means it is incorporated in CO2 molecules and used to track metabolic pathways.
The location of the electron affects how the atom will react with other elements. When electrons are in the lowest available energy level, they are in the ground state. When they absorb energy, they move to a higher energy level, entering the excited state. For instance, when chlorophyll absorbs light energy, electrons within it are boosted to higher energy levels. This provides the energy necessary to produce sugar when they return to their ground state level as they release the energy they absorbed.
Bonding
Elements bond when two nuclei are attracted to each other. Energy is released when a bond is formed. All atoms want to either get rid of all their electrons on their outer shell or fill their outer shell with 8 (or in hydrogen’s case, 2) electrons, which makes them stable. There are 3 kinds of bonds, but for biochemistry, Ionic and covalent bonds are what is relevant.
Ionic bonds form ions (hence the name.) They occur when electrons are transferred. The atom that gains electrons becomes a negatively charged anion. The atom that loses electrons becomes a positively charged cation.
Covalent bonds are made when electrons are shared. This occurs when the two atoms have electronegativities that are closer together than in an ionic bond. Electronegativity is the tendency of an atom to pull electrons towards it. These bonds can be polar if the electronegativity is high enough. A polar molecule is a molecule with a partial charge. For example, water is a polar molecule, as oxygen is extremely electronegative, and water is partially electronegative.

Hydrogen Bonding
Hydrogen bonding is a specific kind of intermolecular force that is essential to life. It is what keeps the 2 strands of DNA bonded together, and gives water its unique characteristics. Since oxygen has a partial negative charge, and hydrogen has a partial positive charge, they are naturally drawn to each other.

Hydrophobic vs Hydrophilic
Polar molecules are hydrophilic. This is because they are attracted to the partially charged ends of water. Hydrophilic means they are attracted to water. (Not in that way… sick) NaCl or table salt is hydrophilic. This is why salt dissolves in water.
Non-polar molecules are hydrophobic. This means they are repelled by water. (They’re filthy water haters.) Lipids are hydrophobic, which is why fats and oils do not dissolve in water.
The cell membrane is a phospholipid bilayer, only allowing nonpolar substances to dissolve through it. Large polar molecules have to use specific hydrophilic channels.
Characteristics of Water
Water is a unique molecule, and without its unique properties, life on earth would not exist as it does, or even at all.
Water has a high specific heat: Because hydrogen bonds are so strong, it requires a lot of heat energy to break them. This is why large bodies of water remain the same temperature, and why coastal cities have a consistent temperature because the water absorbs all the heat energy before it can warm up.
Water has a high heat of vaporisation: A large amount of energy is needed for water to vaporise, which is why sweating is such an effective cooling method.
Water has high adhesion properties: Adhesion is when one substance clings to another. Adhesion causes capillary action, which occurs in the xylem of plants, and is used to bring water up from the roots without expending energy.
Water is a universal solvent: Due to its high polarity, water makes an excellent solvent.
Water is extremely cohesive: Molecules of water tend to stick to each other. This is observed in surface tension and allows for small insects to run across the surface of the water. Cohesion is also necessary to bring water up from the roots, by transpirational-pull cohesion tension.
Ice is less dense than water: Instead of freezing all the way through, ice crystallises, leaving large amounts of space, causing ice to float. This is essential for the survival of marine life during the winter, as they can live beneath the ice.
pH
pH is calculated by taking the -log of the chance of finding hydronium (H30+) ions within a certain amount of water. Hydronium is made in rare circumstances, where a hydrogen ion breaks off from a water molecule. Normally, there is a 1 in 10 million chance of there being a hydronium ion. This is the equivalent of 1x10^-7. The -log of this number is 7, the neutral pH.
Any pH below 7 is acidic. Any pH above 7 is basic. Stomach acid has a pH of 2, while bleach has a pH of 11. Human blood has a pH of around 7.4
Most living cells need to have an internal environment with a pH of around 7. Buffers exist to regulate pH by either absorbing excess hydrogen ions or donating missing hydrogen ions. In human blood, the bicarbonate ion (HCO3) is essential.
Macromolecules
There are 4 types of macromolecules: carbohydrates, lipids, proteins, and nucleic acids.
Carbohydrates
Carbohydrates are made of carbon, hydrogen, and oxygen. They supply quick and easy energy. 1 gram of all carbohydrates will release 4 calories of energy. In our diet, they can be found almost everywhere in foods such as rice, pasta, bread, cookies, etc.
There are 3 kinds of carbohydrates: monosaccharides, disaccharides, and polysaccharides.
Monosaccharides
All monosaccharides have a chemical formula of C6H12O6. It is the placement of the carbon, oxygen, and hydrogen that determines its properties. Glucose, fructose, and galactose are all examples. They are isomers, meaning they have the same chemical formula, but a different structure.

Disaccharides
When 2 monosaccharides join together, they create disaccharides. They all have the chemical formula C12H22O11. Dehydration synthesis is the process that creates them. This process releases 1 molecule of water, hence the name. Lactose, maltose, and sucrose are all examples.
Hydrolysis is the exact opposite of dehydration synthesis. It is used during digestion. One molecule of water is used to breakdown polymers into monomers.
Polysaccharides Polysaccharides are long polymers of carbohydrates. Cellulose (plant cell wall), chitin (exoskeleton, fungi cell wall), glycogen (how animals store carbohydrates) and starch (how plants store carbohydrates) are all examples.
Lipids
Lipids include fats, oils, and waxes. Most contain 1 glycerol and 3 fatty acids. Glycerol is alcohol.

Fatty acids are the building blocks of lipids and are hydrocarbon chains with carboxyl groups at the end. There are 2 varieties; saturated and unsaturated. (3 if you count trans-fats when extra hydrogen is added to the fat to make the lipid solid)
Saturated fats are solid at room temperature, and are famously unhealthy as they are linked to heart disease.
Unsaturated fats are liquid at room temperature and are good dietary fats.

Lipids store much more energy than carbohydrates. 1 gram of any lipid will release 9 calories of heat per gram. They can be structural, as in the phospholipids of the cell membrane, or they can be hormones.
Proteins
Proteins are polymers of amino acids linked together by peptide bonds.
Amino acids are identifiable by their carboxyl group, amine group, and variable R, attached to a central carbon atom.
Proteins are complex and perform a vast array of duties, such as growth and repair, being enzymes, membrane channels, and hormones.
1 gram of protein releases 4 calories of heat.
Proteins contain the elements C H O N P S
There are only 20 amino acids coding for the thousands of proteins in the human body.
Protein Structure
There are 4 levels to the structure of a protein.
The primary structure results from the sequence of amino acids making up the polypeptide
The secondary structure results from hydrogen bonding within the molecule. This causes a helical structure
The tertiary structure is an intricate 3-dimensional shape or conformation of a protein and most directly decides the function of the protein. Enzymes denature in high temperatures or in the wrong pH because the tertiary structure is compromised.
The quaternary structure is only found in proteins that have more than 1 polypeptide chain, such as in haemoglobin.

Enzymes
Enzymes are large proteins
Enzymes lower the energy of activation, speeding up the reaction, as it lowers the amount of energy needed to start the reaction.
The chemical an enzyme works on is known as a substrate
Enzymes are specifically designed for specific substrates. For example, lactase only works on lactose. Notice the naming pattern for enzymes and their substrates.
The induced fit model is an explanation for how they work. When the substrate enters the active site, it induces the enzyme to change its shape to fit the substrate.
Enzymes can be reused as they do not degrade during a reaction
Enzymes are assisted by cofactors (minerals) or coenzymes (vitamins)



Prions
Prions are proteins that cause diseases. Mad cow disease is an example. It is a misformed protein able to influence other proteins to fold in the same way.
Nucleic Acids
There are 2 kinds of nucleic acids: RNA and DNA. They are necessary for carrying genetic information.
Nucleic acids are polymers of nucleotides
The nucleotides are the two purines: Adenine and Guanine, and the 3 pyrimidines, Thymine, Uracil, and Cytosine. Uracil is only found in RNA, and thymine is only found in DNA. Adenine connects with thymine/uracil, and guanine connects with cytosine.


Slice of Life
Water: Making a Splash
You don’t have to be a genius to know that water is essential for life. After all, we’re made up of it, we sweat it, we drink it, some people even opt to give birth in it. But what is it about two hydrogens and an oxygen which make it so sensational?
The answer is to do with water’s structure. A H2O molecule is covalently bonded, which means each atom shares electrons. In this case, the covalent bonds are between two hydrogen atoms and one oxygen atom. Oxygen is cool because it is highly electronegative. Electronegativity is the ability for one atom to “pull” the electrons towards it in a covalent bond. Since oxygen is highly electronegative, it pulls the electrons in the bond towards it which gives the oxygen a slight negative charge because of the electron proximity. This is represented by δ- (delta negative). The hydrogen is therefore δ+ (delta positive) and has a slight positive charge. Overall, the molecule is said to be polar, or to be dipolar in nature, because there is a difference in charge across the molecule.
Water being a dipole gives it different properties, which you need to know about if you are sitting the AS or A level biology exam.
A quick note on hydrogen bonding…
Being a dipole, water has areas of different charge. When many molecules come together, hydrogen bonds can form between H+ on one molecule and O- on another, shown in the diagram with a dashed line.

It is hydrogen bonds which give water a property called surface tension. Water has a high tendency to ‘stick together’, called cohesion. This is important in water transport through the xylem in later units. Surface tension is a bit like a “skin” because it can allow small organisms to walk along it. It occurs because water molecules on the surface bond to their neighbours much like throughout the whole liquid, but since one side is exposed to air and cannot form hydrogen bonds upwards, they will form stronger ones with the molecules beside them. The net attraction is downwards.
Water is good as a temperature buffer too. Heating a substance makes its particles gain more kinetic energy and therefore the overall temperature rises since particles are moving faster. With water, the temperature doesn’t rise as much as other liquids do. This is because it takes more heat energy to raise the temperature of water by 1 degree - it has a high specific heat capacity due to the many hydrogen bonds that have to be broken (even though they are weak on their own). It takes a lot of heat energy for water to raise its temperature significantly.
This is useful in organisms because our cells are mostly water, which can absorb heat energy without raising our temperature very much. Therefore it “buffers” or reduces heat changes. Seas, lakes and oceans are all good environments to live in because they do not change temperature as quickly as air. Aquatic organisms have an environment with less temperature fluctuation than land organisms.
Having a high latent heat of vaporisation means water can cool down organisms by evaporating a small amount of water. Evaporation is when water becomes a gas due to the large amount of KE. Fast-moving molecules are removed when this occurs and take their energy with them, therefore decreasing the amount of energy left behind and cooling it. Sweat is a good example of high latent heat of vaporisation. A small quantity of water is removed with a large cooling effect, meaning temperature is stabilised without losing a lot of water.
Water is also a good solvent (a substance which can dissolve other substances) and this is due to more hydrogen bonding. Water’s charges of H+ and O- are attracted to the positive and negative charges on molecules and therefore solutes such as NaCl are split into Na+ and Cl-, then spread out. Solvent properties are important in transport (such as blood plasma dissolving glucose, vitamins, urea etc), metabolic reactions, urine production and mineral transportation through the xylem and phloem in plants.
Water molecules can also take place in metabolic reactions. Hydrolysis reactions involve breaking down the covalent bonds between hydrogen and oxygen and making new ones, for example, in digestion. Condensation reactions produce water as a byproduct e.g. the formation of phosphodiester bonds. Water is referred to as a metabolite.
Summary
Water is a dipole due to the slight opposite charges on oxygen and hydrogen atoms.
Hydrogen bonds form between hydrogens on one water molecule and oxygens on another.
Because of this, water has the tendency to stick to itself - cohesion. Cohesion is the reason for surface tension.
Water is a good temperature buffer because of its high specific heat capacity. It takes a lot of energy to raise the temperature by a degree.
Water has a high latent heat of vaporisation so evaporating a little has a large cooling effect.
Water is a good solvent because of how the hydrogen bonds attract charged molecules and separate them. This is useful for transporting solutions.
Water is a metabolite important for hydrolysis reactions and produced in condensation reactions.
Happy studying!


Follow @productive-tips for more tips and content like this posted daily! Handpicked and curated with love :)
Polarity, Resonance, and Electron Pushing: Crash Course Organic Chemistry #10:
We’ve all heard the phrase “opposites attract.” It may or may not be true for people, but it’s definitely true in organic chemistry. In this episode of Crash Course Organic Chemistry, we’re learning about electronegativity, polarity, resonance structures, and resonance hybrids. We’ll practice a very important skill for this course that will help us avoid a lot of memorization in the future: electron pushing. It’ll be a lot of trial and error at first, but we all start somewhere!
-
 amateurchemstudent reblogged this · 4 years ago
amateurchemstudent reblogged this · 4 years ago -
 amateurchemstudent liked this · 4 years ago
amateurchemstudent liked this · 4 years ago -
 frozen-possum liked this · 4 years ago
frozen-possum liked this · 4 years ago -
 itiredwriter liked this · 4 years ago
itiredwriter liked this · 4 years ago -
 le-petit-alouette reblogged this · 4 years ago
le-petit-alouette reblogged this · 4 years ago -
 miscellaniousfeminismandactivism reblogged this · 4 years ago
miscellaniousfeminismandactivism reblogged this · 4 years ago -
 eternallytravelling liked this · 4 years ago
eternallytravelling liked this · 4 years ago -
 shadowrebellion liked this · 4 years ago
shadowrebellion liked this · 4 years ago -
 more-punk-pebble liked this · 4 years ago
more-punk-pebble liked this · 4 years ago -
 thexploress liked this · 4 years ago
thexploress liked this · 4 years ago -
 honeyitallreadydid liked this · 4 years ago
honeyitallreadydid liked this · 4 years ago -
 pinklemonboy liked this · 4 years ago
pinklemonboy liked this · 4 years ago -
 solangelo3088 reblogged this · 4 years ago
solangelo3088 reblogged this · 4 years ago -
 solangelo3088 liked this · 4 years ago
solangelo3088 liked this · 4 years ago -
 clatterbane liked this · 4 years ago
clatterbane liked this · 4 years ago -
 availablenever reblogged this · 4 years ago
availablenever reblogged this · 4 years ago -
 availablenever liked this · 4 years ago
availablenever liked this · 4 years ago -
 lazyaroace liked this · 4 years ago
lazyaroace liked this · 4 years ago -
 floatingpandamonster liked this · 4 years ago
floatingpandamonster liked this · 4 years ago -
 cadaverchan liked this · 4 years ago
cadaverchan liked this · 4 years ago -
 angry-emmy liked this · 4 years ago
angry-emmy liked this · 4 years ago -
 just-a-weird-person liked this · 4 years ago
just-a-weird-person liked this · 4 years ago -
 equinexia liked this · 4 years ago
equinexia liked this · 4 years ago -
 vordemtodgefeit liked this · 4 years ago
vordemtodgefeit liked this · 4 years ago -
 littleashmedai liked this · 4 years ago
littleashmedai liked this · 4 years ago -
 emergencytoothpaste reblogged this · 4 years ago
emergencytoothpaste reblogged this · 4 years ago -
 oneflewoverthecuckoos liked this · 4 years ago
oneflewoverthecuckoos liked this · 4 years ago -
 mxmaxs-asorted-things liked this · 4 years ago
mxmaxs-asorted-things liked this · 4 years ago -
 ghostofyou liked this · 4 years ago
ghostofyou liked this · 4 years ago -
 starbitches liked this · 4 years ago
starbitches liked this · 4 years ago -
 peloblancophoto liked this · 4 years ago
peloblancophoto liked this · 4 years ago -
 femmefantome liked this · 4 years ago
femmefantome liked this · 4 years ago -
 narcissasdaffodil reblogged this · 4 years ago
narcissasdaffodil reblogged this · 4 years ago -
 carebearofriddles liked this · 4 years ago
carebearofriddles liked this · 4 years ago -
 narcissasdaffodil liked this · 4 years ago
narcissasdaffodil liked this · 4 years ago -
 childhood-boyfriend liked this · 4 years ago
childhood-boyfriend liked this · 4 years ago -
 uchihasasukeofficial reblogged this · 4 years ago
uchihasasukeofficial reblogged this · 4 years ago -
 itstotaltrash reblogged this · 4 years ago
itstotaltrash reblogged this · 4 years ago -
 worthlessclericbuild liked this · 4 years ago
worthlessclericbuild liked this · 4 years ago -
 rayvenreayes liked this · 4 years ago
rayvenreayes liked this · 4 years ago -
 sorrellegiance liked this · 4 years ago
sorrellegiance liked this · 4 years ago -
 fettucine-al-pacino liked this · 4 years ago
fettucine-al-pacino liked this · 4 years ago -
 thesocietyfanpage liked this · 4 years ago
thesocietyfanpage liked this · 4 years ago -
 just-a-chem-studyblr reblogged this · 4 years ago
just-a-chem-studyblr reblogged this · 4 years ago -
 onesmoothassendoplasmicreticulum liked this · 4 years ago
onesmoothassendoplasmicreticulum liked this · 4 years ago -
 deglutitionborborygmus reblogged this · 4 years ago
deglutitionborborygmus reblogged this · 4 years ago


