Covalent Bonds: Sharing Is Caring!
Covalent Bonds: Sharing Is Caring!
Welcome to my second out of three posts on bonding - ionic, covalent and metallic. This post also covers the coordinate/ dative bond which I can’t remember if I’ve covered before. Only one more of this series left! Find the others here.
Covalent bonding involves one or more shared pairs of electrons between two atoms. These can be found in simple molecular elements and compounds like CO2 , macromolecular structures like diamond and molecular ions such as ammonium. Covalent bonds mostly occur between non-metals but sometimes metals can form covalent bonds.
Single covalent bonds share just one pair of electrons. Double covalent bonds share two. Triple covalent bonds share three.
Each atom usually provides one electron – unpaired in the orbital – in the bond. The number of unpaired electrons in an atom usually shows how many bonds it can make but sometimes atoms promote electrons to fit in more. Covalent bonds are represented with lines between the atoms – double and triple bonds represented with two and three lines respectively.
Dot and cross diagrams show the arrangement of electrons in covalent bonds. They use dots and crosses to demonstrate that the electrons come from different places and often only the outer shell is shown.

The simple explanation as to how atoms form covalent bonds is that one unpaired electron in the orbital of one atom overlaps with one in another atom. Sometimes atoms promote electrons in the same energy level to form more covalent bonds. For example, if an atom wants to make three covalent bonds but has a full 3s2 shell and a 3p1 shell, it can promote one of its 3s2 electrons so that an electron from the other atoms can fill the 3s shell and pair with the new 3p2 shell.
Sometimes promotion does not occur and that means different compounds can be made such as PCl3 or PCl5.

A lone pair of electrons is a pair of electrons from the same energy sub-level uninvolved in bonding. Sometimes these can form something called a coordinate bond, which contains a shared pair of electrons where both come from one atom. The lone pair of electrons is “donated” into the empty orbital of another atom to form a coordinate bond.

This is an example of a coordinate (sometimes called dative) bond between ammonia and a H+ ion which has an empty orbital. The lone pair on the ammonia overlaps with this H+ ion and donates its electrons. Both electrons come from the ammonia’s lone pair so it is a coordinate bond. This is demonstrated with an arrow. The diagram is missing an overall charge of + on the ammonium ion it produces. Coordinate bonds act the same as covalent bonds.
Once you have your covalent bonds, you need to know about covalent substances and their properties. There are two types of covalent substance: simple covalent (molecular) and macromolecular (giant covalent).
Molecular simply means that the formula for the compound or element describes exactly how many atoms are in one molecule, e.g. H2O. Molecular covalent crystalline substances usually exist as single molecules such as iodine or oxygen. They are usually gases or liquids at room temperature but can be low melting point solids.
Solid molecular covalent solids are crystalline so can be called molecular covalent crystals. Iodine and ice are examples of these. Iodine (shown below) has a regular arrangement which makes it a crystalline substance and water, as ice, has a crystalline structure as well.

The properties of these crystals are that they have low melting points, are very brittle due to the lack of strong bonds holding them together and also do not conduct electricity since no ions are present.
The other kind of covalent substance you need to know is macromolecular. This includes giant covalent structures such as diamond or graphite, which are allotropes of carbon. Non-metallic elements and compounds usually form these crystalline structures with a regular arrangement of atoms.
Allotropes are different forms of the same element in the same physical state.
Diamond is the hardest naturally occurring substance on earth therefore is good for cutting glass and drilling and mining. It has a high melting point due to the many covalent bonds which require a lot of energy to break. Each carbon has four of these bonds joining it to four others in a tetrahedral arrangement with a bond angle of 109.5 degrees and it does not conduct electricity or heat because there are no ions free to move.
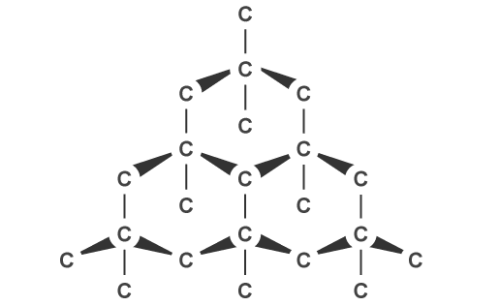
Graphite, on the other hand, can conduct electricity. This is because it has delocalised electrons between the layers which move and carry charge. Carbon atoms within the structure are only bonded to three others in a hexagonal arrangement with a bond angle of 120 degrees. Since only three of carbon’s unpaired electrons are used in bonding, the fourth becomes delocalised and moves between the layers of graphite causing weak attractions, explaining why it can conduct electricity.

Graphite’s layered structure and the weak forces of attractions between it make it a good lubricant and ideal for pencil lead because the layers can slide over each other. The attractions can be broken easily but the covalent bonds within the layers give graphite a high melting point due to the amount of energy needed to break them.
SUMMARY
Covalent bonding involves one or more shared pairs of electrons between two atoms. Covalent bonds mostly occur between non-metals but sometimes metals can form covalent bonds.
Single covalent bonds share just one pair of electrons. Double covalent bonds share two. Triple covalent bonds share three.
Each atom usually provides one electron – unpaired in the orbital – in the bond. The number of unpaired electrons in an atom usually shows how many bonds it can make but sometimes atoms promote electrons to fit in more. Covalent bonds are represented with lines between the atoms.
Dot and cross diagrams use dots and crosses to demonstrate that the electrons come from different places and often only the outer shell is shown.
The simple explanation as to how atoms form covalent bonds is that one unpaired electron in the orbital of one atom overlaps with one in another atom. Sometimes atoms promote electrons in the same energy level to form more covalent bonds.
Sometimes promotion does not occur and that means different compounds can be made such as PCl3 or PCl5.
A lone pair of electrons is a pair of electrons from the same energy sub-level uninvolved in bonding. Sometimes these can form something called a coordinate bond, which contains a shared pair of electrons where both come from one atom. The lone pair of electrons is “donated” into the empty orbital of another atom to form a coordinate bond.
The formation of ammonium is an example of this.
There are two types of covalent substance: simple covalent (molecular) and macromolecular (giant covalent).
Molecular simply means that the formula for the compound or element describes exactly how many atoms are in one molecule, e.g. H2O. Molecular covalent crystalline substances usually exist as single molecules such as iodine or oxygen. They are usually gases or liquids at room temperature but can be low melting point solids.
Solid molecular covalent solids are crystalline so can be called molecular covalent crystals. Iodine and ice are examples of these.
The properties of these crystals are that they have low melting points, are very brittle due to the lack of strong bonds holding them together and also do not conduct electricity since no ions are present.
Giant covalent structures such as diamond or graphite are allotropes of carbon. Allotropes are different forms of the same element in the same physical state.
Diamond has a high melting point due to the many covalent bonds which require a lot of energy to break. Each carbon has four of these bonds joining it to four others in a tetrahedral arrangement with a bond angle of 109.5 degrees and it does not conduct electricity or heat because there are no ions free to move.
Graphite can conduct electricity. This is because it has delocalised electrons between the layers which move and carry charge. Carbon atoms within the structure are only bonded to three others in a hexagonal arrangement with a bond angle of 120 degrees. Since only three of carbon’s unpaired electrons are used in bonding, the fourth becomes delocalised and moves between the layers of graphite causing weak attractions, explaining why it can conduct electricity.
Graphite’s layered structure and the weak forces of attractions between it make it a good lubricant and ideal for pencil lead because the layers can slide over each other. The attractions can be broken easily but the covalent bonds within the layers give graphite a high melting point due to the amount of energy needed to break them.
Happy studying!
More Posts from Amateurchemstudent and Others
It’s World Sleep Day
Log off.
Go back to bed.

i just learned from animal crossing that pondskaters stay on top of the water by secreting an oil from their feet
that seems kinda obvious in hindsight. i always figured they were just, like, light enough to not break surface tension
Metallic Bonding
A short one to finish off my first ever mini-series on bonding – ionic, covalent and finally metallic. There are metallic and metallic compounds and elements but for the A Level exam, we must look at the bonding within metals themselves. Don’t worry – I saved the easiest to last!
Metals are most usually solid so have particles packed close together. These are in layers which mean that the outer electrons can move between them rather than being bound to particular atoms. These are referred to as delocalised electrons because of this.
It’s pretty common knowledge that metals are good conductors of heat and electricity and it’s these delocalised electrons that give them this property.
Metals are therefore without their electrons so become positive ions. The metallic bond is actually the attraction between delocalised electrons and positive metal ions in the lattice. And that’s pretty much metallic bonding, you just need to know the properties of metals which are touched upon at lower levels of education.

These are the properties of metals:
1. High melting points
Metals have large regular structures with strong forces between the oppositely charged positive ions and negative electrons, meaning these must be overcome to melt the metal – this requires a large amount of heat energy. Transition metals tend to have higher melting points than the main group metals because they have large numbers of d-shell electrons which can become delocalised creating a stronger metallic bond. Melting points across a period increase because they can have progressively more delocalised electrons: Na+, Mg 2+ and Al 3+ for example.
2. Heat conductivity
Heat is conducted if particles can move and knock against each other to pass it on. Delocalised electrons allow this to happen. Silver is a particularly good conductor of heat.
3. Electrical conductivity
Delocalised electrons can carry charge and move, the two requirements of electrical conductivity. Current can flow because of these delocalised electrons.
4. Ductile and malleable
Metals can be stretched and hammered into shape, making them ideal for things such as wires. Layered lattices mean that layers can slide over each other without disrupting the bonding – it is all still held together by the delocalised electrons and their strong attraction to the positive metal ions.

5. High densities
Being a solid, metal ions are packed closely together so they have a high density, which makes them ideal for musical instrument strings. These can withstand the frequency of vibration whilst also being thinner.

SUMMARY
Metals are solid so have particles packed close together. These are in layers which mean that the outer electrons can move between them rather than being bound to particular atoms. These are referred to as delocalised electrons because of this.
Metals are therefore without their electrons so become positive ions. The metallic bond is actually the attraction between delocalised electrons and positive metal ions in the lattice.
Metals have high melting points.
Metals have large regular structures with strong forces between the oppositely charged positive ions and negative electrons, meaning these must be overcome to melt the metal – this requires a large amount of heat energy. Transition metals tend to have higher melting points than the main group metals because they have large numbers of d-shell electrons which can become delocalised creating a stronger metallic bond.
Metals conduct heat.
Heat is conducted if particles can move and knock against each other to pass it on. Delocalised electrons allow this to happen.
Metals have good electrical conductivity
Delocalised electrons can carry charge and move, the two requirements of electrical conductivity. Current can flow because of these delocalised electrons.
Metals are ductile and malleable.
Metals can be stretched and hammered into shape, making them ideal for things such as wires. Layered lattices mean that layers can slide over each other without disrupting the bonding – it is all still held together by the delocalised electrons and their strong attraction to the positive metal ions.
Being a solid, metal ions are packed closely together so they have a high density.
Happy studying!

The Name’s Bond ... Ionic Bond.
This is the first in my short series of the three main types of bond - ionic, metallic and covalent. In this, you’ll learn about the properties of the compounds, which atoms they’re found between and how the bonds are formed. Enjoy!
When electrons are transferred from a metal to a non-metal, an ionic compound is formed. Metals usually lose electrons and non-metals usually gain them to get to a noble gas configuration. Transition metals do not always achieve this.
Charged particles that have either lost or gained electrons are called ions and are no longer neutral - metal atoms lose electrons to become positive ions (cations) whereas non-metals gain electrons to become negative ions (anions).
The formation of these ions is usually shown using electron configurations. Make sure you know that the transfer of electrons is not the bond but how the ions are formed.
An ionic bond is the electrostatic attraction between oppositely charged ions.
You need to know how to explain how atoms react with other atoms and for this the electron configurations are needed. You can use dot and cross diagrams for this.

Ionic solids hold ions in 3D structures called ionic lattices. A lattice is a repeating 3D pattern in a crystalline solid. For example, NaCl has a 6:6 arrangement - each Na+ ion is surrounded by 6 Cl- and vice versa.
Ionic solids have many strong electrostatic attractions between their ions. The crystalline shape can be decrepitated (cracked) on heating. Ionic Lattices have high melting and boiling points since they need more energy to break because atoms are held together by lots of strong electrostatic attractions between positive and negative ions. The boiling point of an ionic compound depends on the size of the atomic radius and the charge of the ion. The smaller the ion and the higher the charge, the stronger attraction.

Look at this diagram. It shows how atomic radius decreases across a period regularly. This is not the case with the ions. Positive ions are usually smaller than the atoms they came from because metal atoms lose electrons meaning the nuclear charge increases which draws the electrons closer to the nucleus. For negative ions, they become larger because repulsion between electrons moves them further away - nuclear charge also decreases as more electrons to the same number of protons.
Ionic substances can conduct electricity through the movement of charged particles when molten or dissolved (aqueous). This is because when they are like this, electrons are free to move and carry a charge. Ionic solids cannot conduct electricity.

Ionic compounds are usually soluble in water. This is because the polar water molecules cluster around ions which have broken off the lattice and so separate them from each other. Some substances like aluminium oxide have too strong electrostatic attractions so water cannot break up the lattice - it is insoluble in water.
Molecular ions such as sulfate, nitrate, ammonium or carbonate can exist within ionic compounds. These compounds may have covalent bonds within the ions but overall they are ionic and exhibit thee properties described above.
SUMMARY
When electrons are transferred from a metal to a non-metal, an ionic compound is formed.
Charged particles that have either lost or gained electrons are called ions and are no longer neutral - metal atoms lose electrons to become positive ions (cations) whereas non-metals gain electrons to become negative ions (anions).
The formation of these ions is usually shown using electron configurations. The transfer of electrons is not the bond but how the ions are formed.
An ionic bond is the electrostatic attraction between oppositely charged ions.
Ionic solids hold ions in 3D structures called ionic lattices. A lattice is a repeating 3D pattern in a crystalline solid.
Ionic solids have many strong electrostatic attractions between their ions. The crystalline shape can be decrepitated (cracked) on heating.
Ionic Lattices have high melting and boiling points since they need more energy to break because atoms are held together by lots of strong electrostatic attractions between positive and negative ions.
The boiling point of an ionic compound depends on the size of the atomic radius and the charge of the ion. The smaller the ion and the higher the charge, the stronger attraction.
Positive ions are usually smaller than the atoms they came from because metal atoms lose electrons meaning the nuclear charge increases which draws the electrons closer to the nucleus. Negative ions become larger because repulsion between electrons moves them further away - nuclear charge also decreases as more electrons to the same number of protons.
Ionic substances can conduct electricity through the movement of charged particles when molten or dissolved (aqueous). This is because when they are like this, electrons are free to move and carry a charge. Ionic solids cannot conduct electricity.
Ionic compounds are usually soluble in water because the polar water molecules cluster around ions which have broken off the lattice and so separate them from each other.
Some substances like aluminium oxide have too strong electrostatic attractions so water cannot break up the lattice - it is insoluble in water.
Molecular ions such as sulfate, nitrate, ammonium or carbonate can exist within ionic compounds. These compounds may have covalent bonds within the ions but overall they are ionic and exhibit thee properties described above.
It’s day 5 of #ChemAdvent – here’s why peppermint candy canes make your mouth feel cold! bit.ly/chemadvent2020 https://ift.tt/2JM6bZ7
Haloalkanes and Their Angelic Reactions: Part One
Haloalkanes are more commonly referred to as halogenoalkanes. Obviously you’ve already read my post on halogenoalkanes and their properties so there’s no surprise that you’re itching to read what I’ve got to say about these beauties and their reactions! Should we delve in?
There are a few different kinds of reactions you must learn for the A Level exam that involve halogenoalkanes.
The first is the synthesis of chloroalkanes via the photochemical chlorination of the alkanes. I know it looks scary, but don’t worry, it is simpler than it sounds. It essentially means “forming chloroalkanes through chlorinating an alkane in the presence of sunlight”.
Chlorine will react with methane when UV light is present and will form several kinds of chloroalkanes and fumes of hydrogen chloride gas. Chloromethane was once commonly used as a refridgerant. Depending on how many chlorine molecules there are, there will be different compounds formed:
methane + chlorine -> chloromethane + hydrogen chloride
CH4 + Cl2 -> CH3Cl + HCl
or
methane + chlorine -> trichloromethane + hydrogen chloride
CH4 + 3Cl2 -> CHCl3 + 3HCl
When undergone in real life, mixtures of halogenoalkanes are produced with some long chain alkanes which can be separated out with fractional distillation.
To understand what happens in an overall chemical reaction, chemists use mechanisms. These basically show the step-by-step process that is usually shown by a simple symbol equation that summarises everything.
The chlorination of methane is something you must learn the mechanism for. It’s pretty easy but involves a lot of steps and must be revised periodically to remember them.
The actual reaction is a substitution reaction because one atom or group is replaced by another. Since the chlorine involved is a free radical, it can also be called a free-radical substitution reaction.
1. Initiation
UV light is essential for the first step in the mechanism. This breaks the Cl-Cl covalent bond so that each chlorine leaves with one electron from the shared pair. Chlorine free radicals, with one unpaired electron in the outer shell, are formed. Free radicals are only formed if a bond splits evenly - each atom getting one of the two electrons. The name given to this is homolytic fission.

2. Propagation
This has two sub-steps
(a) Chlorine free radicals (highly reactive) react with methane to form hydrogen chloride and leave a methyl free radical.
Cl• + CH4 -> HCl + •CH3
(b) This free radical then reacts with another chlorine to form chloromethane and another chlorine free radical. Producing free radicals is a chain reaction which is why it is such a problem in ozone depletion - a little amount can cause a lot of destruction.
•CH3 + Cl2 -> CH3Cl + •Cl
3. Termination
This step stops the chain reaction. It only happens when two free radicals collide to form a molecule in several ways:
Cl• + Cl• -> Cl2
UV light would just break down the chlorine molecule again, so although this is technically a termination reaction it is not the most efficient.
Cl• + •CH3 -> CH3Cl
Forming one molecule of methane uses one chlorine and one methyl free radical.
•CH3 + •CH3 -> C2H6
Ethane can be formed from two methyl free radicals - this is why there are longer chain alkanes in the mixture.
This whole process is how organic halogenoalkanes are the product of photochemical reactions of halogens with alkanes in UV light - made via free radical substitution mechanisms in chain reaction.
Another reaction you need to know is a nucleophilic substitution reactions. A nucleophile is an electron pair donor or proton acceptor - the name comes from Greek origins (”loves nucleus”) - such as hydroxide ions, cyanide ions or ammonia molecules. Hydroxide and cyanide ions are negative but ammonia is neutral.

Halogenoalkanes have a polar bond because of the difference between the highly electronegative halogen and the carbon atom. The 𝛿+ carbon can go under nucleophilic attack. The mechanism for negatively charged nucleophiles these in general is:

Nu represents the nucleophile. This example is with a bromoalkane. Make sure to include curly arrows that begin at a lone pair or the centre of a bond and end at an atom or centre of bond, and delta (slight) charges.
Lets look at a more specific example:
One nucleophile that can be used is a hydroxide ion, found in either water or sodium hydroxide. In this case, you need to know about aqueous sodium hydroxide or potassium hydroxide and a halogenoalkane. This takes place at room temperature but is slow so is often refluxed (continuously boiled and condensed back into the reaction flask). Reflux apparatus is shown below:

The halogenoalkane is dissolved into ethanol since it is insoluable in water and this solution along with the aqueous hydroxide can mix. The product produced is an alcohol, which is organic.
The general reaction is:
R-CH2X + NaOH -> CH3CH2OH + NaX
Where X represents a halogen.
You must learn the mechanism for this reaction. The lone pair on the hydroxide attacks the carbon atom attached to the halogen and this causes both carbon electrons to move to the halogen which becomes a halide ion.

The reaction of a hydroxide ion can also be classed as a hydrolysis reaction as it breaks down chemical bonds with water or hydroxide ions. The speed of reaction depends on the strength of the bond - a stronger carbon-halogen bond, a slower reaction.

C-I is the most reactive (reactivity increases down group 7) and C-F is therefore the least reactive and strongest.
Part two of this post will cover nucleophilic substitution of cyanide ions and ammonia molecules, as well as elimination reactions.
SUMMARY
You need to know about the synthesis of chloroalkanes via the photochemical chlorination of the alkanes. - “forming chloroalkanes through chlorinating an alkane in the presence of sunlight”.
Chlorine will react with methane when UV light is present and will form several kinds of chloroalkanes and fumes of hydrogen chloride gas. Depending on how many chlorine molecules there are, there will be different compounds formed.
When undergone in real life, mixtures of halogenoalkanes are produced with some long chain alkanes which can be separated out with fractional distillation.
To understand what happens in an overall chemical reaction, chemists use mechanisms. These basically show the step-by-step process.
The chlorination of methane is something you must learn the mechanism for. The actual reaction is a substitution reaction because one atom or group is replaced by another.
The first step is initiation - UV light is essential for the first step in the mechanism. This breaks the Cl-Cl covalent bond so that each chlorine leaves with one electron from the shared pair. Chlorine free radicals, with one unpaired electron in the outer shell, are formed. Free radicals are only formed if a bond splits evenly - each atom getting one of the two electrons.
Step two is propagation: (a) Chlorine free radicals (highly reactive) react with methane to form hydrogen chloride and leave a methyl free radical (b) this free radical then reacts with another chlorine to form chloromethane and another chlorine free radical. Producing free radicals is a chain reaction which is why it is such a problem in ozone depletion - a little amount can cause a lot of destruction.
To stop the chain reaction, the final step is termination. It only happens when two free radicals collide to form a molecule in several ways: two chlorine free radicals forming a chlorine molecule, two methyl FRs forming ethane or a chlorine FR and a methyl FR forming chloromethane.
Ethane contributes to the longer chain alkanes in the mixture.
Another reaction you need to know is a nucleophilic substitution reactions. A nucleophile is an electron pair donor or proton acceptor, such as hydroxide ions, cyanide ions or ammonia molecules. Hydroxide and cyanide ions are negative but ammonia is neutral.
Halogenoalkanes have a polar bond because of the difference between the highly electronegative halogen and the carbon atom. The 𝛿+ carbon can go under nucleophilic attack.
Nu represents the nucleophile. Make sure to include curly arrows that begin at a lone pair or the centre of a bond and end at an atom or centre of bond, and delta (slight) charges.
One nucleophile that can be used is a hydroxide ion, found in either water or sodium hydroxide. In this case, you need to know about aqueous sodium hydroxide or potassium hydroxide and a halogenoalkane. This takes place at room temperature but is slow so is often refluxed (continuously boiled and condensed back into the reaction flask). The halogenoalkane is dissolved into ethanol since it is insoluable in water and this solution along with the aqueous hydroxide can mix. The product produced is an alcohol, which is organic.
The general reaction is :R-CH2X + NaOH -> CH3CH2OH + NaX where X represents a halogen
The lone pair on the hydroxide attacks the carbon atom attached to the halogen and this causes both carbon electrons to move to the halogen which becomes a halide ion.
The reaction of a hydroxide ion can also be classed as a hydrolysis reaction as it breaks down chemical bonds with water or hydroxide ions.
The speed of reaction depends on the strength of the bond - a stronger carbon-halogen bond, a slower reaction. C-I is the most reactive (reactivity increases down group 7) and C-F is therefore the least reactive and strongest.
-
 svefnsmal liked this · 2 years ago
svefnsmal liked this · 2 years ago -
 number1nerd reblogged this · 3 years ago
number1nerd reblogged this · 3 years ago -
 amateurchemstudent reblogged this · 4 years ago
amateurchemstudent reblogged this · 4 years ago -
 amateurchemstudent liked this · 4 years ago
amateurchemstudent liked this · 4 years ago -
 random-stuff-i-guess liked this · 5 years ago
random-stuff-i-guess liked this · 5 years ago -
 rescuestudies reblogged this · 5 years ago
rescuestudies reblogged this · 5 years ago -
 thesoftestcherub liked this · 5 years ago
thesoftestcherub liked this · 5 years ago -
 khadooy8 reblogged this · 5 years ago
khadooy8 reblogged this · 5 years ago -
 jade12358 liked this · 6 years ago
jade12358 liked this · 6 years ago -
 bioqueen liked this · 6 years ago
bioqueen liked this · 6 years ago -
 the-holistic-stuff liked this · 6 years ago
the-holistic-stuff liked this · 6 years ago -
 dolce-jasmine-blog liked this · 6 years ago
dolce-jasmine-blog liked this · 6 years ago -
 thrteenbeaches reblogged this · 6 years ago
thrteenbeaches reblogged this · 6 years ago -
 standingcastles reblogged this · 6 years ago
standingcastles reblogged this · 6 years ago -
 thefallingdark liked this · 6 years ago
thefallingdark liked this · 6 years ago -
 zephirahredrew liked this · 7 years ago
zephirahredrew liked this · 7 years ago -
 kirbay-zh liked this · 7 years ago
kirbay-zh liked this · 7 years ago -
 electronicstarlightfestival reblogged this · 7 years ago
electronicstarlightfestival reblogged this · 7 years ago -
 chi-coppola96lu755-blog liked this · 7 years ago
chi-coppola96lu755-blog liked this · 7 years ago -
 b4ngchanlover liked this · 7 years ago
b4ngchanlover liked this · 7 years ago -
 theresa1bdbk9-blog liked this · 7 years ago
theresa1bdbk9-blog liked this · 7 years ago -
 poorarthoes reblogged this · 7 years ago
poorarthoes reblogged this · 7 years ago -
 oksanavickers liked this · 7 years ago
oksanavickers liked this · 7 years ago -
 emsstudy-blog liked this · 7 years ago
emsstudy-blog liked this · 7 years ago -
 cherrycokebaby liked this · 7 years ago
cherrycokebaby liked this · 7 years ago -
 blueberry-pancakes-stuff liked this · 7 years ago
blueberry-pancakes-stuff liked this · 7 years ago -
 as-studypeach reblogged this · 7 years ago
as-studypeach reblogged this · 7 years ago -
 sundari67-blog liked this · 7 years ago
sundari67-blog liked this · 7 years ago -
 k2study reblogged this · 7 years ago
k2study reblogged this · 7 years ago -
 mamisgxrl-blog liked this · 7 years ago
mamisgxrl-blog liked this · 7 years ago -
 future-geneius-study liked this · 7 years ago
future-geneius-study liked this · 7 years ago -
 takemetoavalon liked this · 7 years ago
takemetoavalon liked this · 7 years ago -
 malfoyfever-blog reblogged this · 7 years ago
malfoyfever-blog reblogged this · 7 years ago -
 malfoyfever-blog liked this · 7 years ago
malfoyfever-blog liked this · 7 years ago -
 chaoticcspice liked this · 7 years ago
chaoticcspice liked this · 7 years ago -
 stcdy liked this · 7 years ago
stcdy liked this · 7 years ago -
 built-on-dreams-blog liked this · 7 years ago
built-on-dreams-blog liked this · 7 years ago -
 stubborn-studies reblogged this · 7 years ago
stubborn-studies reblogged this · 7 years ago -
 stubborn-studies liked this · 7 years ago
stubborn-studies liked this · 7 years ago -
 quantumskies-blog reblogged this · 7 years ago
quantumskies-blog reblogged this · 7 years ago

