🌻 Little Habits/things To Do More Of 🌻
🌻 little habits/things to do more of 🌻
dailies
make your bed. (no, really.)
set your top 3 to-dos for the day.
do your top 3 to-dos for the day. (heh)
stretch.
unpack your bag when you get home.
prepare your things for the next day before sleeping.
skincare. (your basic cleanse and moisturize)
sweep the floor of your bedroom.
talk to your plants. (if you have plants)
update your financial report/expense tracker.
take a good photo.
meditate.
hug at least three people. (seriously.)
weeklies
polish your school shoes.
mop your bedroom floor.
dare i say, laundry. (don’t put it off!)
exfoliate.
take a leisure walk.
review your past week and plan your next week accordingly. (a part of your routine may not be working–try something new)
make a piece of art. (a sketch, a collage, a quote in pretty lettering, a god-awful poem..)
sanitize your gadgets. (whip out the wet tissue and wipe away at your phone, your laptop, your mouse, your earphones–just don’t forget to IMMEDIATELY follow that up with a dry cloth to prevent fogging and short circuits)
watch a TED Talk.
make a new playlist.
monthlies
wash your bag.
wash your shoes.
change the sheets of your bed and your pillows.
clip your nails. (honestly)
wax/shave. (if you want. i just really like how fresh my skin feels after i torture it with razors and wax strips)
wipe your shelves/the tops of your furniture clean. (try to avoid dusting. it just scatters the dirt everywhere. use a damp cloth instead)
see if there’s anything in your storage that you don’t need/want anymore and give stuff away or sell them.
review your month and plan the next one accordingly. (just like your weeks. remember to refer to your Life Goal/Year’s Goals page)
finish reading at least one book. (and review it!)
discover new songs.
- 🍂
More Posts from Amateurchemstudent and Others
The two kinds of water

Credit: University of Basel
Pre-sorted ortho-water and para-water molecules with differently oriented nuclear spins (blue or red arrows) react with diazenylium ions (centre left) at different speeds.
–
Researchers from the University of Basel’s Department of Chemistry, Switzerland, has investigated how the two forms of water differ in terms of their chemical reactivity – the ability to undergo a chemical reaction. Both forms have almost identical physical properties, which makes their separation particularly challenging.
It is less well-known that water exists in two different forms (isomers) at the molecular level. The difference is in the relative orientation of the nuclear spins of the two hydrogen atoms. Depending on whether the spins are aligned in the same or opposite direction, one refers to ortho- or para-water.
The was made possible by a method based on electric fields. Using this, researchers were able to initiate controlled reactions between the pre-sorted water isomers and ultracold diazenylium ions (protonated nitrogen) held in a trap. During this process, a diazenylium ion transfers its proton to a water molecule. This reaction is also observed in the chemistry of interstellar space.
It was discovered that para-water reacts about 25% faster than ortho-water. This can be explained in terms of the nuclear spin also influencing the rotation of the water molecules. As a result, different attractive forces act between the reaction partners. Para-water is able to attract its reaction partner more strongly than the ortho-form, which leads to an increased chemical reactivity.
Polarity, Resonance, and Electron Pushing: Crash Course Organic Chemistry #10:
We’ve all heard the phrase “opposites attract.” It may or may not be true for people, but it’s definitely true in organic chemistry. In this episode of Crash Course Organic Chemistry, we’re learning about electronegativity, polarity, resonance structures, and resonance hybrids. We’ll practice a very important skill for this course that will help us avoid a lot of memorization in the future: electron pushing. It’ll be a lot of trial and error at first, but we all start somewhere!
Nomenclature - what in the organic chemistry is it?
Organic chemistry is so widely studied it requires a standard system for naming compounds, developed by IUPAC. Nomenclature is simply naming these organic compounds.
So, you want to be an organic chemist? Well, it starts here. Are you ready?
(psst… once you’ve learnt this theory, try a quiz here!)
1. Count your longest continuous chain of carbons.
Bear in mind that some chains may be bent. You’re looking for the longest chain of subsequent carbon atoms. This number correlates to root names that indicate the carbon chain length, listed below:

The second part of naming your base comes from the bonding in the chain. Is it purely single bonds or are there double bonds in there? If you are familiar with carbon chemistry, you’ll know that saturated hydrocarbons are called alkanes and unsaturated hydrocarbons are called alkenes. Therefore, the syllable -ane is used when it has only single bonds and the syllable -ene is used when it has some double bonds. For example:
Sometimes carbon chains exist in rings rather than chains. These have the prefix of -cyclo.
2. Identify your side chains attached to this main carbon and name them.
Side chains are added as prefixes to the root names. Sometimes called substituents, these are basically anything that comes off the carbon chain. Examples of the prefixes are listed below:

There are other prefixes such as fluoro (-F) and chloro (-Cl) which can describe what is coming off the chain.
3. Identify where each side chain is attached and indicate the position by adding a number to the name.
We aim to have numbers as small as possible. For example, if bromine is on the second carbon of a 5-carbon saturated chain, we number it as 2-bromopentane instead of 4-bromopentane, since it would essentially be 2-bromopentane if it was flipped. Locant is the term used for the number which describes the position of the substitute group, e.g. the ‘2′ in 2-chlorobutane is the locant.
Sometimes there are two or more side chains e.g. a methyl group and a chlorine attached to a pentane. In these cases, these rules apply:
1. Names are written alphabetically.
2. A separate number is needed for each side chain or group.
3. Hyphens are used to separate numbers and letters.

This would be named 2-chloro-3-methyl-pentane. This is because the longest chain of carbons is 5 (pentane), the chlorine is on the second carbon (2-chloro) and the methyl group is on the third carbon (3-methyl). It is 2-chloro rather than 4-chloro as we aim to have as small as numbers as possible.
Another variation of this step to be aware of is how many of the same side chains or groups there are, for example, having two methyl groups would be dimethyl rather than solely methyl. Each group must also be given numbers separated by commas to show where each one is located.
The list of these prefixes is found here:

Convention does not usually require mono- to go before a single group or side chain.
4. Number the positions of double bonds if applicable.
Alkenes and other compounds have double bonds. These must be indicated with numbers. For example, pent-2-ene shows that the double bond is between carbon 2 and carbon 3. The number goes in the middle of the original root name e.g. butene, pentene.
(!) Below is a list of functional groups that you may need to study for the AS and A Level chemistry exams. “R” represents misc. carbons. It is important to know that some groups are more prioritised than naming. From the most to least priority: carboxylic acid, ester, acyl chloride, nitrile, aldehyde, ketone, alcohol, amine, alkene, halogenalkane. It is worthwhile learning these.

bigger version here (I suggest downloading and printing it)
But wait, there’s more:
Here are some things to bear in mind when naming organic compounds:
1. The letter ‘e’ is removed when there are two vowels together e.g. propanone rather than propaneone. The ‘e’ isn’t removed when it is next to consonant, e.g. propanenitrile isn’t propannitrile.
2. When compounds contain two different, one is named as part of the unbranched chain and the other is named as a substituent. Which way round this goes depends on the priority.
SUMMARY
Count your longest continuous chain of carbons.
Chains may be bent. You’re looking for the longest chain of subsequent carbon atoms. This number correlates to root names that indicate the carbon chain length, e.g. pentane.
The second part of naming your base comes from the bonding in the chain. Is it purely single bonds or are there double bonds in there? The syllable -ane is used when it has only single bonds and the syllable -ene is used when it has some double bonds.
Rings have the prefix of -cyclo.
Identify your side chains attached to this main carbon and name them.
Side chains are added as prefixes to the root names. Sometimes called substituents, these are basically anything that comes off the carbon chain.
There are other prefixes such as fluoro (-F) and chloro (-Cl) which can describe what is coming off the chain.
Identify where each side chain is attached and indicate the position by adding a number to the name.
We aim to have numbers as small as possible. Locant is the term used for the number which describes the position of the substitute group, e.g. the ‘2′ in 2-chlorobutane is the locant.
Sometimes there are two or more side chains e.g. a methyl group and a chlorine attached to a pentane. In these cases, names are written alphabetically, a separate number is needed for each side chain or group and hyphens are used to separate numbers and letters.
When there are two or more of the same side chains or substituent groups, these must also be given numbers separated by commas to show where each one is located.
Number the positions of double bonds if applicable.
Alkenes and other compounds have double bonds. These must be indicated with numbers. The number goes in the middle of the original root name e.g. butene, pentene.
It is worthwhile learning the other functional groups that can be added on.They have varying priorities.
The letter ‘e’ is removed when there are two vowels together e.g. propanone rather than propaneone. The ‘e’ isn’t removed when it is next to consonant, e.g. propanenitrile isn’t propannitrile.
When compounds contain two different, one is named as part of the unbranched chain and the other is named as a substituent. Which way round this goes depends on the priority.
Happy studying guys!
It’s World Sleep Day
Log off.
Go back to bed.
Covalent Bonds: Sharing Is Caring!
Welcome to my second out of three posts on bonding - ionic, covalent and metallic. This post also covers the coordinate/ dative bond which I can’t remember if I’ve covered before. Only one more of this series left! Find the others here.
Covalent bonding involves one or more shared pairs of electrons between two atoms. These can be found in simple molecular elements and compounds like CO2 , macromolecular structures like diamond and molecular ions such as ammonium. Covalent bonds mostly occur between non-metals but sometimes metals can form covalent bonds.
Single covalent bonds share just one pair of electrons. Double covalent bonds share two. Triple covalent bonds share three.
Each atom usually provides one electron – unpaired in the orbital – in the bond. The number of unpaired electrons in an atom usually shows how many bonds it can make but sometimes atoms promote electrons to fit in more. Covalent bonds are represented with lines between the atoms – double and triple bonds represented with two and three lines respectively.
Dot and cross diagrams show the arrangement of electrons in covalent bonds. They use dots and crosses to demonstrate that the electrons come from different places and often only the outer shell is shown.

The simple explanation as to how atoms form covalent bonds is that one unpaired electron in the orbital of one atom overlaps with one in another atom. Sometimes atoms promote electrons in the same energy level to form more covalent bonds. For example, if an atom wants to make three covalent bonds but has a full 3s2 shell and a 3p1 shell, it can promote one of its 3s2 electrons so that an electron from the other atoms can fill the 3s shell and pair with the new 3p2 shell.
Sometimes promotion does not occur and that means different compounds can be made such as PCl3 or PCl5.

A lone pair of electrons is a pair of electrons from the same energy sub-level uninvolved in bonding. Sometimes these can form something called a coordinate bond, which contains a shared pair of electrons where both come from one atom. The lone pair of electrons is “donated” into the empty orbital of another atom to form a coordinate bond.

This is an example of a coordinate (sometimes called dative) bond between ammonia and a H+ ion which has an empty orbital. The lone pair on the ammonia overlaps with this H+ ion and donates its electrons. Both electrons come from the ammonia’s lone pair so it is a coordinate bond. This is demonstrated with an arrow. The diagram is missing an overall charge of + on the ammonium ion it produces. Coordinate bonds act the same as covalent bonds.
Once you have your covalent bonds, you need to know about covalent substances and their properties. There are two types of covalent substance: simple covalent (molecular) and macromolecular (giant covalent).
Molecular simply means that the formula for the compound or element describes exactly how many atoms are in one molecule, e.g. H2O. Molecular covalent crystalline substances usually exist as single molecules such as iodine or oxygen. They are usually gases or liquids at room temperature but can be low melting point solids.
Solid molecular covalent solids are crystalline so can be called molecular covalent crystals. Iodine and ice are examples of these. Iodine (shown below) has a regular arrangement which makes it a crystalline substance and water, as ice, has a crystalline structure as well.

The properties of these crystals are that they have low melting points, are very brittle due to the lack of strong bonds holding them together and also do not conduct electricity since no ions are present.
The other kind of covalent substance you need to know is macromolecular. This includes giant covalent structures such as diamond or graphite, which are allotropes of carbon. Non-metallic elements and compounds usually form these crystalline structures with a regular arrangement of atoms.
Allotropes are different forms of the same element in the same physical state.
Diamond is the hardest naturally occurring substance on earth therefore is good for cutting glass and drilling and mining. It has a high melting point due to the many covalent bonds which require a lot of energy to break. Each carbon has four of these bonds joining it to four others in a tetrahedral arrangement with a bond angle of 109.5 degrees and it does not conduct electricity or heat because there are no ions free to move.
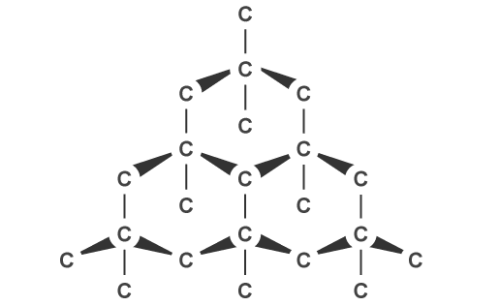
Graphite, on the other hand, can conduct electricity. This is because it has delocalised electrons between the layers which move and carry charge. Carbon atoms within the structure are only bonded to three others in a hexagonal arrangement with a bond angle of 120 degrees. Since only three of carbon’s unpaired electrons are used in bonding, the fourth becomes delocalised and moves between the layers of graphite causing weak attractions, explaining why it can conduct electricity.

Graphite’s layered structure and the weak forces of attractions between it make it a good lubricant and ideal for pencil lead because the layers can slide over each other. The attractions can be broken easily but the covalent bonds within the layers give graphite a high melting point due to the amount of energy needed to break them.
SUMMARY
Covalent bonding involves one or more shared pairs of electrons between two atoms. Covalent bonds mostly occur between non-metals but sometimes metals can form covalent bonds.
Single covalent bonds share just one pair of electrons. Double covalent bonds share two. Triple covalent bonds share three.
Each atom usually provides one electron – unpaired in the orbital – in the bond. The number of unpaired electrons in an atom usually shows how many bonds it can make but sometimes atoms promote electrons to fit in more. Covalent bonds are represented with lines between the atoms.
Dot and cross diagrams use dots and crosses to demonstrate that the electrons come from different places and often only the outer shell is shown.
The simple explanation as to how atoms form covalent bonds is that one unpaired electron in the orbital of one atom overlaps with one in another atom. Sometimes atoms promote electrons in the same energy level to form more covalent bonds.
Sometimes promotion does not occur and that means different compounds can be made such as PCl3 or PCl5.
A lone pair of electrons is a pair of electrons from the same energy sub-level uninvolved in bonding. Sometimes these can form something called a coordinate bond, which contains a shared pair of electrons where both come from one atom. The lone pair of electrons is “donated” into the empty orbital of another atom to form a coordinate bond.
The formation of ammonium is an example of this.
There are two types of covalent substance: simple covalent (molecular) and macromolecular (giant covalent).
Molecular simply means that the formula for the compound or element describes exactly how many atoms are in one molecule, e.g. H2O. Molecular covalent crystalline substances usually exist as single molecules such as iodine or oxygen. They are usually gases or liquids at room temperature but can be low melting point solids.
Solid molecular covalent solids are crystalline so can be called molecular covalent crystals. Iodine and ice are examples of these.
The properties of these crystals are that they have low melting points, are very brittle due to the lack of strong bonds holding them together and also do not conduct electricity since no ions are present.
Giant covalent structures such as diamond or graphite are allotropes of carbon. Allotropes are different forms of the same element in the same physical state.
Diamond has a high melting point due to the many covalent bonds which require a lot of energy to break. Each carbon has four of these bonds joining it to four others in a tetrahedral arrangement with a bond angle of 109.5 degrees and it does not conduct electricity or heat because there are no ions free to move.
Graphite can conduct electricity. This is because it has delocalised electrons between the layers which move and carry charge. Carbon atoms within the structure are only bonded to three others in a hexagonal arrangement with a bond angle of 120 degrees. Since only three of carbon’s unpaired electrons are used in bonding, the fourth becomes delocalised and moves between the layers of graphite causing weak attractions, explaining why it can conduct electricity.
Graphite’s layered structure and the weak forces of attractions between it make it a good lubricant and ideal for pencil lead because the layers can slide over each other. The attractions can be broken easily but the covalent bonds within the layers give graphite a high melting point due to the amount of energy needed to break them.
Happy studying!

Slice of Life
Breaking Down Alkanes - isn’t it cracking?
Unfortunately, if you’re sitting your A Level chemistry exam, you need to know a little more than the basic properties of alkanes outlined in my last post. Luckily though, this post takes you through fractional distillation and the two types of cracking - isn’t that convenient?
Crude oil contains carbon compounds formed by the effects of pressure and high temperature on plant and animal remnants. It is viscious, black and found in rocks beneath the earth’s surface. It is a mixture of mainly alkane hydrocarbons which are separated by a process called fractional distillation. Crude oil is essential because it is burned as a fuel and each fraction has different properties e.g. diesel, petrol, jet fuel.
Fractional distillation is the continual evaporation and condensation of a mixture which causes fractions to split due to a difference in boiling point. It is important to note that fractional distillation does not separate crude oil into pure compounds but rather less complex mixtures. Fractions are groups of compounds that have similar boiling points and are removed at the same level of a fractionating column.
The first step in this process is to heat crude oil in a furnace until some changes state from a liquid to a vapour. This mixture goes up a fractionating tower or column which is hotter at the bottom than the top and reaches a layer which is cool enough to condense and be collected. Shorter chain molecules are collected at the top where it is cooler since they have lower boiling points.

As you go down the fractionating column, bear in mind that: the column temperature increases, the boiling point increases, the number of carbon atoms increases and the strength of the Van der Waals’ between molecules increases.
Different fractions have different usefulnesses and often, it is the fractions with lower boiling points and shorter chains which are much more purposeful. Therefore there needs to be a process to getting shorter chains because they are the least abundant in crude oil samples. To meet demand, long chain molecules that are less useful are broken down into shorter chain molecules. This is done by cracking.
Cracking is a process where long chain hydrocarbon molecules are broken down into shorter chain molecules which are in high demand. This can be done one of two ways - thermal or catalytic.
Thermal cracking involves heating long chain alkanes to high temperatures - usually between 1000 - 1200K. It also uses high pressures up to 70atm and takes just one second. It only needs a second because the conditions could decompose the molecule completely to produce carbon and hydrogen instead. The conditions produce shorter chain alkanes and mostly alkenes.
A typical equation for this:

Decane -> octane + ethene
C10H22 -> C8H18 + C2H4
Catalytic cracking also breaks down long alkanes by heat under pressure using the presence of a zeolite catalyst. Temperature used is approx. 800-1000K and the pressure is often between 1-2 atm. Zeolite is an acidic mineral with a honeycomb structure, made from aluminium oxide and silicion dioxide. The honeycomb structure gives the catalyst a larger surface area which increases ROR. Factories which catalytically crack are often operated continuously for around 3 years at a time and produce branched alkanes, cycloalkanes and aromatic compounds.
You need to be able to compare the conditions of catalytic and thermal cracking for the A Level exam. Know that thermal cracking has a high temperature and pressure, a short duration, no catalyst and produces a high percentage of alkenes and some short chain alkanes. Catalytic uses a catalyst, a high temperature, a low pressure and produces aromatic hydrocarbons and motor fuels.
SUMMARY
Crude oil contains carbon compounds formed by the effects of pressure and high temperature on plant and animal remnants. I It is a mixture of mainly alkane hydrocarbons which are separated by a process called fractional distillation.
Fractional distillation is the continual evaporation and condensation of a mixture which causes fractions to split due to a difference in boiling point.
It is important to note that fractional distillation does not separate crude oil into pure compounds but rather less complex mixtures.
Fractions are groups of compounds that have similar boiling points and are removed at the same level of a fractionating column.
The first step in this process is to heat crude oil in a furnace until some changes state from a liquid to a vapour. This mixture goes up a fractionating tower or column which is hotter at the bottom than the top and reaches a layer which is cool enough to condense and be collected. Shorter chain molecules are collected at the top where it is cooler since they have lower boiling points.
As you go down the fractionating column, bear in mind that: the column temperature increases, the boiling point increases, the number of carbon atoms increases and the strength of the Van der Waals’ between molecules increases.
Fractions with lower boiling points and shorter chains are much more purposeful but are the least abundant in crude oil samples. To meet demand, long chain molecules that are less useful are broken down into shorter chain molecules.
Cracking is a process where long chain hydrocarbon molecules are broken down into shorter chain molecules which are in high demand.
Thermal cracking involves heating long chain alkanes to high temperatures - usually between 1000 - 1200K. It also uses high pressures up to 70atm and takes just one second. It only needs a second because the conditions could decompose the molecule completely to produce carbon and hydrogen instead. The conditions produce shorter chain alkanes and mostly alkenes.
Catalytic cracking also breaks down long alkanes by heat under pressure using the presence of a zeolite catalyst. Temperature used is approx. 800-1000K and the pressure is often between 1-2 atm. Zeolite is an acidic mineral with a honeycomb structure, made from aluminium oxide and silicion dioxide. The honeycomb structure gives the catalyst a larger surface area which increases ROR.
You need to be able to compare the conditions of catalytic and thermal cracking for the A Level exam. Know that thermal cracking has a high temperature and pressure, a short duration, no catalyst and produces a high percentage of alkenes and some short chain alkanes. Catalytic uses a catalyst, a high temperature, a low pressure and produces aromatic hydrocarbons and motor fuels.
Happy studying!
Universities are like "we can't accept everyone based on accepted grades because we gave too many offers out." They give out too many offers because they're horrified at the thought that they might end up with too many empty places on courses, so they oversubscribe so they can get those sweet sweet tuition fees.
Just in case anyone thought here was a thing that Tony Blair had no hand in, for once.
-
 d-i-r-t-y-p-i-c-t-u-r-e liked this · 3 months ago
d-i-r-t-y-p-i-c-t-u-r-e liked this · 3 months ago -
 naniixte liked this · 4 months ago
naniixte liked this · 4 months ago -
 curlytopp liked this · 5 months ago
curlytopp liked this · 5 months ago -
 soon--soon liked this · 5 months ago
soon--soon liked this · 5 months ago -
 moss-gender liked this · 5 months ago
moss-gender liked this · 5 months ago -
 nefarious-virgo liked this · 5 months ago
nefarious-virgo liked this · 5 months ago -
 eternalcelestialchaos reblogged this · 6 months ago
eternalcelestialchaos reblogged this · 6 months ago -
 papasfritasthings liked this · 6 months ago
papasfritasthings liked this · 6 months ago -
 ghost-breathes reblogged this · 6 months ago
ghost-breathes reblogged this · 6 months ago -
 panthepancake liked this · 7 months ago
panthepancake liked this · 7 months ago -
 wreathofsmoke reblogged this · 7 months ago
wreathofsmoke reblogged this · 7 months ago -
 wreathofsmoke liked this · 7 months ago
wreathofsmoke liked this · 7 months ago -
 a-gemstonegentleman reblogged this · 8 months ago
a-gemstonegentleman reblogged this · 8 months ago -
 thelandofararat liked this · 8 months ago
thelandofararat liked this · 8 months ago -
 drooliarae liked this · 9 months ago
drooliarae liked this · 9 months ago -
 teacupnoir liked this · 10 months ago
teacupnoir liked this · 10 months ago -
 eternalcelestialchaos liked this · 10 months ago
eternalcelestialchaos liked this · 10 months ago -
 worldofffdreams liked this · 10 months ago
worldofffdreams liked this · 10 months ago -
 fruitingb0dy reblogged this · 10 months ago
fruitingb0dy reblogged this · 10 months ago -
 fruitingb0dy liked this · 10 months ago
fruitingb0dy liked this · 10 months ago -
 hey-bbgs reblogged this · 11 months ago
hey-bbgs reblogged this · 11 months ago -
 mylifeasawarriorforchrist liked this · 1 year ago
mylifeasawarriorforchrist liked this · 1 year ago -
 ischariot liked this · 1 year ago
ischariot liked this · 1 year ago -
 theiaphaessa reblogged this · 1 year ago
theiaphaessa reblogged this · 1 year ago -
 theiaphaessa liked this · 1 year ago
theiaphaessa liked this · 1 year ago -
 thetemplesofisis liked this · 1 year ago
thetemplesofisis liked this · 1 year ago -
 sunfortune liked this · 1 year ago
sunfortune liked this · 1 year ago -
 nuu-wa reblogged this · 1 year ago
nuu-wa reblogged this · 1 year ago -
 citrondemiel reblogged this · 1 year ago
citrondemiel reblogged this · 1 year ago -
 citrondemiel liked this · 1 year ago
citrondemiel liked this · 1 year ago -
 sporadicdreamersuitcaserascal liked this · 1 year ago
sporadicdreamersuitcaserascal liked this · 1 year ago -
 bambinalove liked this · 1 year ago
bambinalove liked this · 1 year ago -
 larrydog reblogged this · 1 year ago
larrydog reblogged this · 1 year ago -
 imnotyoshi reblogged this · 1 year ago
imnotyoshi reblogged this · 1 year ago -
 agirlinthegalaxy reblogged this · 1 year ago
agirlinthegalaxy reblogged this · 1 year ago -
 alfsanpol liked this · 1 year ago
alfsanpol liked this · 1 year ago -
 laribot liked this · 1 year ago
laribot liked this · 1 year ago -
 scarlettmarieharbor liked this · 1 year ago
scarlettmarieharbor liked this · 1 year ago -
 fondlymorning reblogged this · 1 year ago
fondlymorning reblogged this · 1 year ago -
 dynamicsbitch reblogged this · 1 year ago
dynamicsbitch reblogged this · 1 year ago -
 muhtesemz liked this · 1 year ago
muhtesemz liked this · 1 year ago -
 dayfairies2 reblogged this · 1 year ago
dayfairies2 reblogged this · 1 year ago


