Me When Tetravalence

me when tetravalence
More Posts from Amateurchemstudent and Others
Haloalkanes and Their Angelic Reactions: Part One
Haloalkanes are more commonly referred to as halogenoalkanes. Obviously you’ve already read my post on halogenoalkanes and their properties so there’s no surprise that you’re itching to read what I’ve got to say about these beauties and their reactions! Should we delve in?
There are a few different kinds of reactions you must learn for the A Level exam that involve halogenoalkanes.
The first is the synthesis of chloroalkanes via the photochemical chlorination of the alkanes. I know it looks scary, but don’t worry, it is simpler than it sounds. It essentially means “forming chloroalkanes through chlorinating an alkane in the presence of sunlight”.
Chlorine will react with methane when UV light is present and will form several kinds of chloroalkanes and fumes of hydrogen chloride gas. Chloromethane was once commonly used as a refridgerant. Depending on how many chlorine molecules there are, there will be different compounds formed:
methane + chlorine -> chloromethane + hydrogen chloride
CH4 + Cl2 -> CH3Cl + HCl
or
methane + chlorine -> trichloromethane + hydrogen chloride
CH4 + 3Cl2 -> CHCl3 + 3HCl
When undergone in real life, mixtures of halogenoalkanes are produced with some long chain alkanes which can be separated out with fractional distillation.
To understand what happens in an overall chemical reaction, chemists use mechanisms. These basically show the step-by-step process that is usually shown by a simple symbol equation that summarises everything.
The chlorination of methane is something you must learn the mechanism for. It’s pretty easy but involves a lot of steps and must be revised periodically to remember them.
The actual reaction is a substitution reaction because one atom or group is replaced by another. Since the chlorine involved is a free radical, it can also be called a free-radical substitution reaction.
1. Initiation
UV light is essential for the first step in the mechanism. This breaks the Cl-Cl covalent bond so that each chlorine leaves with one electron from the shared pair. Chlorine free radicals, with one unpaired electron in the outer shell, are formed. Free radicals are only formed if a bond splits evenly - each atom getting one of the two electrons. The name given to this is homolytic fission.

2. Propagation
This has two sub-steps
(a) Chlorine free radicals (highly reactive) react with methane to form hydrogen chloride and leave a methyl free radical.
Cl• + CH4 -> HCl + •CH3
(b) This free radical then reacts with another chlorine to form chloromethane and another chlorine free radical. Producing free radicals is a chain reaction which is why it is such a problem in ozone depletion - a little amount can cause a lot of destruction.
•CH3 + Cl2 -> CH3Cl + •Cl
3. Termination
This step stops the chain reaction. It only happens when two free radicals collide to form a molecule in several ways:
Cl• + Cl• -> Cl2
UV light would just break down the chlorine molecule again, so although this is technically a termination reaction it is not the most efficient.
Cl• + •CH3 -> CH3Cl
Forming one molecule of methane uses one chlorine and one methyl free radical.
•CH3 + •CH3 -> C2H6
Ethane can be formed from two methyl free radicals - this is why there are longer chain alkanes in the mixture.
This whole process is how organic halogenoalkanes are the product of photochemical reactions of halogens with alkanes in UV light - made via free radical substitution mechanisms in chain reaction.
Another reaction you need to know is a nucleophilic substitution reactions. A nucleophile is an electron pair donor or proton acceptor - the name comes from Greek origins (”loves nucleus”) - such as hydroxide ions, cyanide ions or ammonia molecules. Hydroxide and cyanide ions are negative but ammonia is neutral.

Halogenoalkanes have a polar bond because of the difference between the highly electronegative halogen and the carbon atom. The 𝛿+ carbon can go under nucleophilic attack. The mechanism for negatively charged nucleophiles these in general is:

Nu represents the nucleophile. This example is with a bromoalkane. Make sure to include curly arrows that begin at a lone pair or the centre of a bond and end at an atom or centre of bond, and delta (slight) charges.
Lets look at a more specific example:
One nucleophile that can be used is a hydroxide ion, found in either water or sodium hydroxide. In this case, you need to know about aqueous sodium hydroxide or potassium hydroxide and a halogenoalkane. This takes place at room temperature but is slow so is often refluxed (continuously boiled and condensed back into the reaction flask). Reflux apparatus is shown below:

The halogenoalkane is dissolved into ethanol since it is insoluable in water and this solution along with the aqueous hydroxide can mix. The product produced is an alcohol, which is organic.
The general reaction is:
R-CH2X + NaOH -> CH3CH2OH + NaX
Where X represents a halogen.
You must learn the mechanism for this reaction. The lone pair on the hydroxide attacks the carbon atom attached to the halogen and this causes both carbon electrons to move to the halogen which becomes a halide ion.

The reaction of a hydroxide ion can also be classed as a hydrolysis reaction as it breaks down chemical bonds with water or hydroxide ions. The speed of reaction depends on the strength of the bond - a stronger carbon-halogen bond, a slower reaction.

C-I is the most reactive (reactivity increases down group 7) and C-F is therefore the least reactive and strongest.
Part two of this post will cover nucleophilic substitution of cyanide ions and ammonia molecules, as well as elimination reactions.
SUMMARY
You need to know about the synthesis of chloroalkanes via the photochemical chlorination of the alkanes. - “forming chloroalkanes through chlorinating an alkane in the presence of sunlight”.
Chlorine will react with methane when UV light is present and will form several kinds of chloroalkanes and fumes of hydrogen chloride gas. Depending on how many chlorine molecules there are, there will be different compounds formed.
When undergone in real life, mixtures of halogenoalkanes are produced with some long chain alkanes which can be separated out with fractional distillation.
To understand what happens in an overall chemical reaction, chemists use mechanisms. These basically show the step-by-step process.
The chlorination of methane is something you must learn the mechanism for. The actual reaction is a substitution reaction because one atom or group is replaced by another.
The first step is initiation - UV light is essential for the first step in the mechanism. This breaks the Cl-Cl covalent bond so that each chlorine leaves with one electron from the shared pair. Chlorine free radicals, with one unpaired electron in the outer shell, are formed. Free radicals are only formed if a bond splits evenly - each atom getting one of the two electrons.
Step two is propagation: (a) Chlorine free radicals (highly reactive) react with methane to form hydrogen chloride and leave a methyl free radical (b) this free radical then reacts with another chlorine to form chloromethane and another chlorine free radical. Producing free radicals is a chain reaction which is why it is such a problem in ozone depletion - a little amount can cause a lot of destruction.
To stop the chain reaction, the final step is termination. It only happens when two free radicals collide to form a molecule in several ways: two chlorine free radicals forming a chlorine molecule, two methyl FRs forming ethane or a chlorine FR and a methyl FR forming chloromethane.
Ethane contributes to the longer chain alkanes in the mixture.
Another reaction you need to know is a nucleophilic substitution reactions. A nucleophile is an electron pair donor or proton acceptor, such as hydroxide ions, cyanide ions or ammonia molecules. Hydroxide and cyanide ions are negative but ammonia is neutral.
Halogenoalkanes have a polar bond because of the difference between the highly electronegative halogen and the carbon atom. The 𝛿+ carbon can go under nucleophilic attack.
Nu represents the nucleophile. Make sure to include curly arrows that begin at a lone pair or the centre of a bond and end at an atom or centre of bond, and delta (slight) charges.
One nucleophile that can be used is a hydroxide ion, found in either water or sodium hydroxide. In this case, you need to know about aqueous sodium hydroxide or potassium hydroxide and a halogenoalkane. This takes place at room temperature but is slow so is often refluxed (continuously boiled and condensed back into the reaction flask). The halogenoalkane is dissolved into ethanol since it is insoluable in water and this solution along with the aqueous hydroxide can mix. The product produced is an alcohol, which is organic.
The general reaction is :R-CH2X + NaOH -> CH3CH2OH + NaX where X represents a halogen
The lone pair on the hydroxide attacks the carbon atom attached to the halogen and this causes both carbon electrons to move to the halogen which becomes a halide ion.
The reaction of a hydroxide ion can also be classed as a hydrolysis reaction as it breaks down chemical bonds with water or hydroxide ions.
The speed of reaction depends on the strength of the bond - a stronger carbon-halogen bond, a slower reaction. C-I is the most reactive (reactivity increases down group 7) and C-F is therefore the least reactive and strongest.

Plenty of opportunities to wear sunglasses this week! 😎 Here’s the science behind how the protect your eyes from the sun’s UV radiation in C&EN: https://ift.tt/2XW7h8L https://ift.tt/3gT8PI6

Today is #InternationalMakeUpDay! Here’s a graphic looking at the various components of nail polish 💅 https://ift.tt/32fnwAh https://ift.tt/3jWclTk
Biochemistry
Update: Pictures are working!
Atoms
There are a few basic chemistry concepts that are essential to understand. For starters, understanding what an atom is and its basic properties.
Atoms are the building block of all matter. They have a positive nucleus, with positive protons, and neutral neutrons. In a large area surrounding the nucleus, is the electron cloud, made of negatively charged electrons.
An atom in its elemental state is always neutral.
When an element has a charge, it is because it has an unequal number of protons an electrons, making it an ion. Sometimes an element’s nucleus has an unequal number of neutrons and protons, making it an isotope. Carbon-14, for example, has 8 neutrons, instead of the 6 that Carbon-12 has. Carbon-14 is also a radioisotope, meaning it emits particles and decays at a rate called a half-life, making it useful for fossil dating. Along with that, radioactive carbon can be used as a tracer. This means it is incorporated in CO2 molecules and used to track metabolic pathways.
The location of the electron affects how the atom will react with other elements. When electrons are in the lowest available energy level, they are in the ground state. When they absorb energy, they move to a higher energy level, entering the excited state. For instance, when chlorophyll absorbs light energy, electrons within it are boosted to higher energy levels. This provides the energy necessary to produce sugar when they return to their ground state level as they release the energy they absorbed.
Bonding
Elements bond when two nuclei are attracted to each other. Energy is released when a bond is formed. All atoms want to either get rid of all their electrons on their outer shell or fill their outer shell with 8 (or in hydrogen’s case, 2) electrons, which makes them stable. There are 3 kinds of bonds, but for biochemistry, Ionic and covalent bonds are what is relevant.
Ionic bonds form ions (hence the name.) They occur when electrons are transferred. The atom that gains electrons becomes a negatively charged anion. The atom that loses electrons becomes a positively charged cation.
Covalent bonds are made when electrons are shared. This occurs when the two atoms have electronegativities that are closer together than in an ionic bond. Electronegativity is the tendency of an atom to pull electrons towards it. These bonds can be polar if the electronegativity is high enough. A polar molecule is a molecule with a partial charge. For example, water is a polar molecule, as oxygen is extremely electronegative, and water is partially electronegative.

Hydrogen Bonding
Hydrogen bonding is a specific kind of intermolecular force that is essential to life. It is what keeps the 2 strands of DNA bonded together, and gives water its unique characteristics. Since oxygen has a partial negative charge, and hydrogen has a partial positive charge, they are naturally drawn to each other.

Hydrophobic vs Hydrophilic
Polar molecules are hydrophilic. This is because they are attracted to the partially charged ends of water. Hydrophilic means they are attracted to water. (Not in that way… sick) NaCl or table salt is hydrophilic. This is why salt dissolves in water.
Non-polar molecules are hydrophobic. This means they are repelled by water. (They’re filthy water haters.) Lipids are hydrophobic, which is why fats and oils do not dissolve in water.
The cell membrane is a phospholipid bilayer, only allowing nonpolar substances to dissolve through it. Large polar molecules have to use specific hydrophilic channels.
Characteristics of Water
Water is a unique molecule, and without its unique properties, life on earth would not exist as it does, or even at all.
Water has a high specific heat: Because hydrogen bonds are so strong, it requires a lot of heat energy to break them. This is why large bodies of water remain the same temperature, and why coastal cities have a consistent temperature because the water absorbs all the heat energy before it can warm up.
Water has a high heat of vaporisation: A large amount of energy is needed for water to vaporise, which is why sweating is such an effective cooling method.
Water has high adhesion properties: Adhesion is when one substance clings to another. Adhesion causes capillary action, which occurs in the xylem of plants, and is used to bring water up from the roots without expending energy.
Water is a universal solvent: Due to its high polarity, water makes an excellent solvent.
Water is extremely cohesive: Molecules of water tend to stick to each other. This is observed in surface tension and allows for small insects to run across the surface of the water. Cohesion is also necessary to bring water up from the roots, by transpirational-pull cohesion tension.
Ice is less dense than water: Instead of freezing all the way through, ice crystallises, leaving large amounts of space, causing ice to float. This is essential for the survival of marine life during the winter, as they can live beneath the ice.
pH
pH is calculated by taking the -log of the chance of finding hydronium (H30+) ions within a certain amount of water. Hydronium is made in rare circumstances, where a hydrogen ion breaks off from a water molecule. Normally, there is a 1 in 10 million chance of there being a hydronium ion. This is the equivalent of 1x10^-7. The -log of this number is 7, the neutral pH.
Any pH below 7 is acidic. Any pH above 7 is basic. Stomach acid has a pH of 2, while bleach has a pH of 11. Human blood has a pH of around 7.4
Most living cells need to have an internal environment with a pH of around 7. Buffers exist to regulate pH by either absorbing excess hydrogen ions or donating missing hydrogen ions. In human blood, the bicarbonate ion (HCO3) is essential.
Macromolecules
There are 4 types of macromolecules: carbohydrates, lipids, proteins, and nucleic acids.
Carbohydrates
Carbohydrates are made of carbon, hydrogen, and oxygen. They supply quick and easy energy. 1 gram of all carbohydrates will release 4 calories of energy. In our diet, they can be found almost everywhere in foods such as rice, pasta, bread, cookies, etc.
There are 3 kinds of carbohydrates: monosaccharides, disaccharides, and polysaccharides.
Monosaccharides
All monosaccharides have a chemical formula of C6H12O6. It is the placement of the carbon, oxygen, and hydrogen that determines its properties. Glucose, fructose, and galactose are all examples. They are isomers, meaning they have the same chemical formula, but a different structure.

Disaccharides
When 2 monosaccharides join together, they create disaccharides. They all have the chemical formula C12H22O11. Dehydration synthesis is the process that creates them. This process releases 1 molecule of water, hence the name. Lactose, maltose, and sucrose are all examples.
Hydrolysis is the exact opposite of dehydration synthesis. It is used during digestion. One molecule of water is used to breakdown polymers into monomers.
Polysaccharides Polysaccharides are long polymers of carbohydrates. Cellulose (plant cell wall), chitin (exoskeleton, fungi cell wall), glycogen (how animals store carbohydrates) and starch (how plants store carbohydrates) are all examples.
Lipids
Lipids include fats, oils, and waxes. Most contain 1 glycerol and 3 fatty acids. Glycerol is alcohol.

Fatty acids are the building blocks of lipids and are hydrocarbon chains with carboxyl groups at the end. There are 2 varieties; saturated and unsaturated. (3 if you count trans-fats when extra hydrogen is added to the fat to make the lipid solid)
Saturated fats are solid at room temperature, and are famously unhealthy as they are linked to heart disease.
Unsaturated fats are liquid at room temperature and are good dietary fats.

Lipids store much more energy than carbohydrates. 1 gram of any lipid will release 9 calories of heat per gram. They can be structural, as in the phospholipids of the cell membrane, or they can be hormones.
Proteins
Proteins are polymers of amino acids linked together by peptide bonds.
Amino acids are identifiable by their carboxyl group, amine group, and variable R, attached to a central carbon atom.
Proteins are complex and perform a vast array of duties, such as growth and repair, being enzymes, membrane channels, and hormones.
1 gram of protein releases 4 calories of heat.
Proteins contain the elements C H O N P S
There are only 20 amino acids coding for the thousands of proteins in the human body.
Protein Structure
There are 4 levels to the structure of a protein.
The primary structure results from the sequence of amino acids making up the polypeptide
The secondary structure results from hydrogen bonding within the molecule. This causes a helical structure
The tertiary structure is an intricate 3-dimensional shape or conformation of a protein and most directly decides the function of the protein. Enzymes denature in high temperatures or in the wrong pH because the tertiary structure is compromised.
The quaternary structure is only found in proteins that have more than 1 polypeptide chain, such as in haemoglobin.

Enzymes
Enzymes are large proteins
Enzymes lower the energy of activation, speeding up the reaction, as it lowers the amount of energy needed to start the reaction.
The chemical an enzyme works on is known as a substrate
Enzymes are specifically designed for specific substrates. For example, lactase only works on lactose. Notice the naming pattern for enzymes and their substrates.
The induced fit model is an explanation for how they work. When the substrate enters the active site, it induces the enzyme to change its shape to fit the substrate.
Enzymes can be reused as they do not degrade during a reaction
Enzymes are assisted by cofactors (minerals) or coenzymes (vitamins)



Prions
Prions are proteins that cause diseases. Mad cow disease is an example. It is a misformed protein able to influence other proteins to fold in the same way.
Nucleic Acids
There are 2 kinds of nucleic acids: RNA and DNA. They are necessary for carrying genetic information.
Nucleic acids are polymers of nucleotides
The nucleotides are the two purines: Adenine and Guanine, and the 3 pyrimidines, Thymine, Uracil, and Cytosine. Uracil is only found in RNA, and thymine is only found in DNA. Adenine connects with thymine/uracil, and guanine connects with cytosine.

Nomenclature - what in the organic chemistry is it?
Organic chemistry is so widely studied it requires a standard system for naming compounds, developed by IUPAC. Nomenclature is simply naming these organic compounds.
So, you want to be an organic chemist? Well, it starts here. Are you ready?
(psst… once you’ve learnt this theory, try a quiz here!)
1. Count your longest continuous chain of carbons.
Bear in mind that some chains may be bent. You’re looking for the longest chain of subsequent carbon atoms. This number correlates to root names that indicate the carbon chain length, listed below:

The second part of naming your base comes from the bonding in the chain. Is it purely single bonds or are there double bonds in there? If you are familiar with carbon chemistry, you’ll know that saturated hydrocarbons are called alkanes and unsaturated hydrocarbons are called alkenes. Therefore, the syllable -ane is used when it has only single bonds and the syllable -ene is used when it has some double bonds. For example:
Sometimes carbon chains exist in rings rather than chains. These have the prefix of -cyclo.
2. Identify your side chains attached to this main carbon and name them.
Side chains are added as prefixes to the root names. Sometimes called substituents, these are basically anything that comes off the carbon chain. Examples of the prefixes are listed below:

There are other prefixes such as fluoro (-F) and chloro (-Cl) which can describe what is coming off the chain.
3. Identify where each side chain is attached and indicate the position by adding a number to the name.
We aim to have numbers as small as possible. For example, if bromine is on the second carbon of a 5-carbon saturated chain, we number it as 2-bromopentane instead of 4-bromopentane, since it would essentially be 2-bromopentane if it was flipped. Locant is the term used for the number which describes the position of the substitute group, e.g. the ‘2′ in 2-chlorobutane is the locant.
Sometimes there are two or more side chains e.g. a methyl group and a chlorine attached to a pentane. In these cases, these rules apply:
1. Names are written alphabetically.
2. A separate number is needed for each side chain or group.
3. Hyphens are used to separate numbers and letters.

This would be named 2-chloro-3-methyl-pentane. This is because the longest chain of carbons is 5 (pentane), the chlorine is on the second carbon (2-chloro) and the methyl group is on the third carbon (3-methyl). It is 2-chloro rather than 4-chloro as we aim to have as small as numbers as possible.
Another variation of this step to be aware of is how many of the same side chains or groups there are, for example, having two methyl groups would be dimethyl rather than solely methyl. Each group must also be given numbers separated by commas to show where each one is located.
The list of these prefixes is found here:

Convention does not usually require mono- to go before a single group or side chain.
4. Number the positions of double bonds if applicable.
Alkenes and other compounds have double bonds. These must be indicated with numbers. For example, pent-2-ene shows that the double bond is between carbon 2 and carbon 3. The number goes in the middle of the original root name e.g. butene, pentene.
(!) Below is a list of functional groups that you may need to study for the AS and A Level chemistry exams. “R” represents misc. carbons. It is important to know that some groups are more prioritised than naming. From the most to least priority: carboxylic acid, ester, acyl chloride, nitrile, aldehyde, ketone, alcohol, amine, alkene, halogenalkane. It is worthwhile learning these.

bigger version here (I suggest downloading and printing it)
But wait, there’s more:
Here are some things to bear in mind when naming organic compounds:
1. The letter ‘e’ is removed when there are two vowels together e.g. propanone rather than propaneone. The ‘e’ isn’t removed when it is next to consonant, e.g. propanenitrile isn’t propannitrile.
2. When compounds contain two different, one is named as part of the unbranched chain and the other is named as a substituent. Which way round this goes depends on the priority.
SUMMARY
Count your longest continuous chain of carbons.
Chains may be bent. You’re looking for the longest chain of subsequent carbon atoms. This number correlates to root names that indicate the carbon chain length, e.g. pentane.
The second part of naming your base comes from the bonding in the chain. Is it purely single bonds or are there double bonds in there? The syllable -ane is used when it has only single bonds and the syllable -ene is used when it has some double bonds.
Rings have the prefix of -cyclo.
Identify your side chains attached to this main carbon and name them.
Side chains are added as prefixes to the root names. Sometimes called substituents, these are basically anything that comes off the carbon chain.
There are other prefixes such as fluoro (-F) and chloro (-Cl) which can describe what is coming off the chain.
Identify where each side chain is attached and indicate the position by adding a number to the name.
We aim to have numbers as small as possible. Locant is the term used for the number which describes the position of the substitute group, e.g. the ‘2′ in 2-chlorobutane is the locant.
Sometimes there are two or more side chains e.g. a methyl group and a chlorine attached to a pentane. In these cases, names are written alphabetically, a separate number is needed for each side chain or group and hyphens are used to separate numbers and letters.
When there are two or more of the same side chains or substituent groups, these must also be given numbers separated by commas to show where each one is located.
Number the positions of double bonds if applicable.
Alkenes and other compounds have double bonds. These must be indicated with numbers. The number goes in the middle of the original root name e.g. butene, pentene.
It is worthwhile learning the other functional groups that can be added on.They have varying priorities.
The letter ‘e’ is removed when there are two vowels together e.g. propanone rather than propaneone. The ‘e’ isn’t removed when it is next to consonant, e.g. propanenitrile isn’t propannitrile.
When compounds contain two different, one is named as part of the unbranched chain and the other is named as a substituent. Which way round this goes depends on the priority.
Happy studying guys!
Enthalpy - a thermodynamic property
When I first learned about enthalpy, I was shocked - it felt more like a physics lesson than a chemistry lesson. The thought of learning more about thermodynamics than my basic understanding from my many science lessons in lower school made me bored out of my mind. But enthalpy is actually pretty interesting, once you get your head around it…
Reactions which release heat to their surroundings are described to be exothermic. These are reactions like combustion reactions, oxidation reactions and neutralisation reactions. Endothermic reactions take in heat from their surroundings, such as in thermal decomposition. Reversible reactions are endothermic in one direction and exothermic in the other.
These facts are important when you start to look at enthalpy. Enthalpy is basically a thermodynamic property linked to internal energy, represented by a capital H. This is pretty much the energy released in bond breaking and made in bond making. We usually measure a change in enthalpy, represented by ∆H. ∆H = enthalpy of the products (H1) - enthalpy of the reactants (H2). This is because we cannot measure enthalpy directly.
In exothermic reactions, ∆H is negative whereas in endothermic reactions, ∆H is positive.
∆H is always measured under standard conditions of 298K and 100kPa.
In reversible reactions, the ∆H value is the same numerical value forwards and backwards but the sign is reversed. For example, in a forward exothermic reaction, the ∆H value would be -ve but in the backwards reaction (endothermic) the ∆H would be +ve.
Reaction profiles are diagrams of enthalpy levels of reactants and products in a chemical reaction. X axis is enthalpy rather than ∆H and the Y axis is the progress of reaction, reaction coordinate or extent of reaction. Two horizontal lines show the enthalpy of reactants and products with the reactants on the left and the products on the right. These should be labelled with their names or formulae.
In an endothermic reaction, product lines are higher enthalpy values than reactants. In an exothermic reaction, product lines are lower enthalpy values than reactants. The difference between product and reactant lines is labelled as ∆H. Values are measured in kJ mol-1.
Reaction pathways are shown with lines from the reactants to the products on enthalpy level diagrams. This shows the “journey” that the enthalpy takes during a reaction. They require an input of energy to break bonds before new bonds can form the products. The activation energy is the peak of the pathway above the enthalpy of reactants. It is the minimum amount of energy that reactants must have to react.

Standard enthalpy values are the ∆H values for enthalpy changes of specific reactions measured under standard conditions, represented by ⊖. There are three of these:
1. Standard enthalpy of reaction ( ΔHr⊖ )
The enthalpy change when substances react under standard conditions in quantities given by the equation for the reaction.
2. Standard enthalpy of formation ( ΔfH⊖ )
The enthalpy change when 1 mole of a compound is formed from its constitutent elements with all reactants and products in standard states under standard conditions.
The enthalpy of formation for an element is zero is it is in it’s standard state for example, O2 enthalpy is zero.
3. Standard enthalpy of combustion ( ΔcH⊖ )
The enthalpy change when 1 mole of a substance is burned completely in excess oxygen with all reactants and products in their standard states under standard conditions.
Values for standard enthalpy of formation and combustion must be kept to per mole of what they refer.
Summary
Reactions which release heat to their surroundings are described to be exothermic. Endothermic reactions take in heat from their surroundings, such as in thermal decomposition.
Reversible reactions are endothermic in one direction and exothermic in the other.
Enthalpy is a thermodynamic property linked to internal energy, represented by a capital H. We usually measure a change in enthalpy, represented by ∆H.
∆H = enthalpy of the products (H1) - enthalpy of the reactants (H2). We cannot measure enthalpy directly.
In exothermic reactions, ∆H is negative whereas in endothermic reactions, ∆H is positive.
∆H is always measured under standard conditions of 298K and 100kPa.
In reversible reactions, the ∆H value is the same numerical value forwards and backwards but the sign is reversed.
Reaction profiles are diagrams of enthalpy levels of reactants and products in a chemical reaction. They
In an endothermic reaction, product lines are higher enthalpy values than reactants. In an exothermic reaction, product lines are lower enthalpy values than reactants.
The difference between product and reactant lines is labelled as ∆H.
Values are measured in kJ mol-1.
Reaction pathways are shown with lines from the reactants to the products on enthalpy level diagrams. They plot enthalpy against reaction progress.
Reactions require an input of energy to break bonds before new bonds can form the products. The activation energy is the peak of the pathway above the enthalpy of reactants. It is the minimum amount of energy that reactants must have to react.
Standard enthalpy values are the ∆H values for enthalpy changes of specific reactions measured under standard conditions, represented by ⊖.
Standard enthalpy of reaction ( ΔHr⊖ ) is the enthalpy change when substances react under standard conditions in quantities given by the equation for the reaction.
Standard enthalpy of formation ( ΔfH⊖ ) is the enthalpy change when 1 mole of a compound is formed from its constitutent elements with all reactants and products in standard states under standard conditions.
The enthalpy of formation for an element is zero is it is in it’s standard state.
Standard enthalpy of combustion ( ΔcH⊖ ) is the enthalpy change when 1 mole of a substance is burned completely in excess oxygen with all reactants and products in their standard states under standard conditions.
Values for standard enthalpy of formation and combustion must be kept to per mole of what they refer.
Happy studying!
Covalent Bonds: Sharing Is Caring!
Welcome to my second out of three posts on bonding - ionic, covalent and metallic. This post also covers the coordinate/ dative bond which I can’t remember if I’ve covered before. Only one more of this series left! Find the others here.
Covalent bonding involves one or more shared pairs of electrons between two atoms. These can be found in simple molecular elements and compounds like CO2 , macromolecular structures like diamond and molecular ions such as ammonium. Covalent bonds mostly occur between non-metals but sometimes metals can form covalent bonds.
Single covalent bonds share just one pair of electrons. Double covalent bonds share two. Triple covalent bonds share three.
Each atom usually provides one electron – unpaired in the orbital – in the bond. The number of unpaired electrons in an atom usually shows how many bonds it can make but sometimes atoms promote electrons to fit in more. Covalent bonds are represented with lines between the atoms – double and triple bonds represented with two and three lines respectively.
Dot and cross diagrams show the arrangement of electrons in covalent bonds. They use dots and crosses to demonstrate that the electrons come from different places and often only the outer shell is shown.

The simple explanation as to how atoms form covalent bonds is that one unpaired electron in the orbital of one atom overlaps with one in another atom. Sometimes atoms promote electrons in the same energy level to form more covalent bonds. For example, if an atom wants to make three covalent bonds but has a full 3s2 shell and a 3p1 shell, it can promote one of its 3s2 electrons so that an electron from the other atoms can fill the 3s shell and pair with the new 3p2 shell.
Sometimes promotion does not occur and that means different compounds can be made such as PCl3 or PCl5.

A lone pair of electrons is a pair of electrons from the same energy sub-level uninvolved in bonding. Sometimes these can form something called a coordinate bond, which contains a shared pair of electrons where both come from one atom. The lone pair of electrons is “donated” into the empty orbital of another atom to form a coordinate bond.

This is an example of a coordinate (sometimes called dative) bond between ammonia and a H+ ion which has an empty orbital. The lone pair on the ammonia overlaps with this H+ ion and donates its electrons. Both electrons come from the ammonia’s lone pair so it is a coordinate bond. This is demonstrated with an arrow. The diagram is missing an overall charge of + on the ammonium ion it produces. Coordinate bonds act the same as covalent bonds.
Once you have your covalent bonds, you need to know about covalent substances and their properties. There are two types of covalent substance: simple covalent (molecular) and macromolecular (giant covalent).
Molecular simply means that the formula for the compound or element describes exactly how many atoms are in one molecule, e.g. H2O. Molecular covalent crystalline substances usually exist as single molecules such as iodine or oxygen. They are usually gases or liquids at room temperature but can be low melting point solids.
Solid molecular covalent solids are crystalline so can be called molecular covalent crystals. Iodine and ice are examples of these. Iodine (shown below) has a regular arrangement which makes it a crystalline substance and water, as ice, has a crystalline structure as well.

The properties of these crystals are that they have low melting points, are very brittle due to the lack of strong bonds holding them together and also do not conduct electricity since no ions are present.
The other kind of covalent substance you need to know is macromolecular. This includes giant covalent structures such as diamond or graphite, which are allotropes of carbon. Non-metallic elements and compounds usually form these crystalline structures with a regular arrangement of atoms.
Allotropes are different forms of the same element in the same physical state.
Diamond is the hardest naturally occurring substance on earth therefore is good for cutting glass and drilling and mining. It has a high melting point due to the many covalent bonds which require a lot of energy to break. Each carbon has four of these bonds joining it to four others in a tetrahedral arrangement with a bond angle of 109.5 degrees and it does not conduct electricity or heat because there are no ions free to move.
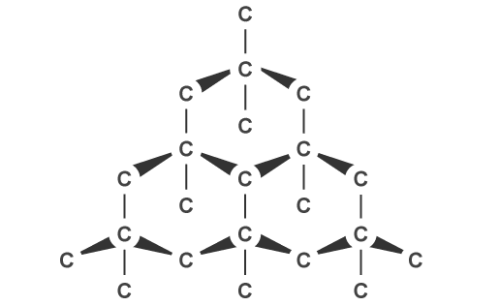
Graphite, on the other hand, can conduct electricity. This is because it has delocalised electrons between the layers which move and carry charge. Carbon atoms within the structure are only bonded to three others in a hexagonal arrangement with a bond angle of 120 degrees. Since only three of carbon’s unpaired electrons are used in bonding, the fourth becomes delocalised and moves between the layers of graphite causing weak attractions, explaining why it can conduct electricity.

Graphite’s layered structure and the weak forces of attractions between it make it a good lubricant and ideal for pencil lead because the layers can slide over each other. The attractions can be broken easily but the covalent bonds within the layers give graphite a high melting point due to the amount of energy needed to break them.
SUMMARY
Covalent bonding involves one or more shared pairs of electrons between two atoms. Covalent bonds mostly occur between non-metals but sometimes metals can form covalent bonds.
Single covalent bonds share just one pair of electrons. Double covalent bonds share two. Triple covalent bonds share three.
Each atom usually provides one electron – unpaired in the orbital – in the bond. The number of unpaired electrons in an atom usually shows how many bonds it can make but sometimes atoms promote electrons to fit in more. Covalent bonds are represented with lines between the atoms.
Dot and cross diagrams use dots and crosses to demonstrate that the electrons come from different places and often only the outer shell is shown.
The simple explanation as to how atoms form covalent bonds is that one unpaired electron in the orbital of one atom overlaps with one in another atom. Sometimes atoms promote electrons in the same energy level to form more covalent bonds.
Sometimes promotion does not occur and that means different compounds can be made such as PCl3 or PCl5.
A lone pair of electrons is a pair of electrons from the same energy sub-level uninvolved in bonding. Sometimes these can form something called a coordinate bond, which contains a shared pair of electrons where both come from one atom. The lone pair of electrons is “donated” into the empty orbital of another atom to form a coordinate bond.
The formation of ammonium is an example of this.
There are two types of covalent substance: simple covalent (molecular) and macromolecular (giant covalent).
Molecular simply means that the formula for the compound or element describes exactly how many atoms are in one molecule, e.g. H2O. Molecular covalent crystalline substances usually exist as single molecules such as iodine or oxygen. They are usually gases or liquids at room temperature but can be low melting point solids.
Solid molecular covalent solids are crystalline so can be called molecular covalent crystals. Iodine and ice are examples of these.
The properties of these crystals are that they have low melting points, are very brittle due to the lack of strong bonds holding them together and also do not conduct electricity since no ions are present.
Giant covalent structures such as diamond or graphite are allotropes of carbon. Allotropes are different forms of the same element in the same physical state.
Diamond has a high melting point due to the many covalent bonds which require a lot of energy to break. Each carbon has four of these bonds joining it to four others in a tetrahedral arrangement with a bond angle of 109.5 degrees and it does not conduct electricity or heat because there are no ions free to move.
Graphite can conduct electricity. This is because it has delocalised electrons between the layers which move and carry charge. Carbon atoms within the structure are only bonded to three others in a hexagonal arrangement with a bond angle of 120 degrees. Since only three of carbon’s unpaired electrons are used in bonding, the fourth becomes delocalised and moves between the layers of graphite causing weak attractions, explaining why it can conduct electricity.
Graphite’s layered structure and the weak forces of attractions between it make it a good lubricant and ideal for pencil lead because the layers can slide over each other. The attractions can be broken easily but the covalent bonds within the layers give graphite a high melting point due to the amount of energy needed to break them.
Happy studying!
-
 pdfbabe liked this · 3 years ago
pdfbabe liked this · 3 years ago -
 probabilities liked this · 3 years ago
probabilities liked this · 3 years ago -
 gigipiet13 liked this · 3 years ago
gigipiet13 liked this · 3 years ago -
 avatarofthebeholding liked this · 4 years ago
avatarofthebeholding liked this · 4 years ago -
 crystal-isaac liked this · 4 years ago
crystal-isaac liked this · 4 years ago -
 amateurchemstudent reblogged this · 4 years ago
amateurchemstudent reblogged this · 4 years ago -
 amateurchemstudent liked this · 4 years ago
amateurchemstudent liked this · 4 years ago -
 jo-unsolved liked this · 4 years ago
jo-unsolved liked this · 4 years ago -
 scaredandbored reblogged this · 4 years ago
scaredandbored reblogged this · 4 years ago -
 scaredandbored liked this · 4 years ago
scaredandbored liked this · 4 years ago -
 hanhan5545 liked this · 4 years ago
hanhan5545 liked this · 4 years ago -
 breekonandhope-deliveries liked this · 4 years ago
breekonandhope-deliveries liked this · 4 years ago -
 bluebyrd-screaming reblogged this · 4 years ago
bluebyrd-screaming reblogged this · 4 years ago -
 ilovelilies liked this · 4 years ago
ilovelilies liked this · 4 years ago -
 melissamelimimi liked this · 4 years ago
melissamelimimi liked this · 4 years ago -
 pepis-benis liked this · 4 years ago
pepis-benis liked this · 4 years ago -
 lnane reblogged this · 4 years ago
lnane reblogged this · 4 years ago -
 stpamique reblogged this · 4 years ago
stpamique reblogged this · 4 years ago -
 stpamique liked this · 4 years ago
stpamique liked this · 4 years ago -
 helloangrydestiny reblogged this · 4 years ago
helloangrydestiny reblogged this · 4 years ago -
 helloangrydestiny liked this · 4 years ago
helloangrydestiny liked this · 4 years ago -
 pdfbabe reblogged this · 4 years ago
pdfbabe reblogged this · 4 years ago -
 intearsaboutrobots liked this · 4 years ago
intearsaboutrobots liked this · 4 years ago -
 ducktoothcollection reblogged this · 4 years ago
ducktoothcollection reblogged this · 4 years ago -
 iconocat liked this · 4 years ago
iconocat liked this · 4 years ago -
 sofflepoffle liked this · 4 years ago
sofflepoffle liked this · 4 years ago -
 jestergirlbosom reblogged this · 4 years ago
jestergirlbosom reblogged this · 4 years ago -
 jestergirlbosom liked this · 4 years ago
jestergirlbosom liked this · 4 years ago -
 pdfbabe reblogged this · 4 years ago
pdfbabe reblogged this · 4 years ago -
 megamentelover liked this · 4 years ago
megamentelover liked this · 4 years ago -
 nora-the-explorer liked this · 4 years ago
nora-the-explorer liked this · 4 years ago -
 waste-mixture liked this · 4 years ago
waste-mixture liked this · 4 years ago -
 mirai-ccl-blog liked this · 4 years ago
mirai-ccl-blog liked this · 4 years ago -
 kel-mine reblogged this · 4 years ago
kel-mine reblogged this · 4 years ago -
 kel-mine liked this · 4 years ago
kel-mine liked this · 4 years ago -
 zaydeen reblogged this · 4 years ago
zaydeen reblogged this · 4 years ago -
 f3mm3dyk3 liked this · 4 years ago
f3mm3dyk3 liked this · 4 years ago -
 multifandomworshipper liked this · 4 years ago
multifandomworshipper liked this · 4 years ago -
 themiddlepagesofabook liked this · 4 years ago
themiddlepagesofabook liked this · 4 years ago -
 actually-i-am-god liked this · 4 years ago
actually-i-am-god liked this · 4 years ago -
 yomannylu liked this · 4 years ago
yomannylu liked this · 4 years ago -
 qtbmx liked this · 4 years ago
qtbmx liked this · 4 years ago -
 thelumpiestpotato liked this · 4 years ago
thelumpiestpotato liked this · 4 years ago -
 critically-unraveled liked this · 4 years ago
critically-unraveled liked this · 4 years ago


