Enthalpy - A Thermodynamic Property
Enthalpy - a thermodynamic property
When I first learned about enthalpy, I was shocked - it felt more like a physics lesson than a chemistry lesson. The thought of learning more about thermodynamics than my basic understanding from my many science lessons in lower school made me bored out of my mind. But enthalpy is actually pretty interesting, once you get your head around it…
Reactions which release heat to their surroundings are described to be exothermic. These are reactions like combustion reactions, oxidation reactions and neutralisation reactions. Endothermic reactions take in heat from their surroundings, such as in thermal decomposition. Reversible reactions are endothermic in one direction and exothermic in the other.
These facts are important when you start to look at enthalpy. Enthalpy is basically a thermodynamic property linked to internal energy, represented by a capital H. This is pretty much the energy released in bond breaking and made in bond making. We usually measure a change in enthalpy, represented by ∆H. ∆H = enthalpy of the products (H1) - enthalpy of the reactants (H2). This is because we cannot measure enthalpy directly.
In exothermic reactions, ∆H is negative whereas in endothermic reactions, ∆H is positive.
∆H is always measured under standard conditions of 298K and 100kPa.
In reversible reactions, the ∆H value is the same numerical value forwards and backwards but the sign is reversed. For example, in a forward exothermic reaction, the ∆H value would be -ve but in the backwards reaction (endothermic) the ∆H would be +ve.
Reaction profiles are diagrams of enthalpy levels of reactants and products in a chemical reaction. X axis is enthalpy rather than ∆H and the Y axis is the progress of reaction, reaction coordinate or extent of reaction. Two horizontal lines show the enthalpy of reactants and products with the reactants on the left and the products on the right. These should be labelled with their names or formulae.
In an endothermic reaction, product lines are higher enthalpy values than reactants. In an exothermic reaction, product lines are lower enthalpy values than reactants. The difference between product and reactant lines is labelled as ∆H. Values are measured in kJ mol-1.
Reaction pathways are shown with lines from the reactants to the products on enthalpy level diagrams. This shows the “journey” that the enthalpy takes during a reaction. They require an input of energy to break bonds before new bonds can form the products. The activation energy is the peak of the pathway above the enthalpy of reactants. It is the minimum amount of energy that reactants must have to react.

Standard enthalpy values are the ∆H values for enthalpy changes of specific reactions measured under standard conditions, represented by ⊖. There are three of these:
1. Standard enthalpy of reaction ( ΔHr⊖ )
The enthalpy change when substances react under standard conditions in quantities given by the equation for the reaction.
2. Standard enthalpy of formation ( ΔfH⊖ )
The enthalpy change when 1 mole of a compound is formed from its constitutent elements with all reactants and products in standard states under standard conditions.
The enthalpy of formation for an element is zero is it is in it’s standard state for example, O2 enthalpy is zero.
3. Standard enthalpy of combustion ( ΔcH⊖ )
The enthalpy change when 1 mole of a substance is burned completely in excess oxygen with all reactants and products in their standard states under standard conditions.
Values for standard enthalpy of formation and combustion must be kept to per mole of what they refer.
Summary
Reactions which release heat to their surroundings are described to be exothermic. Endothermic reactions take in heat from their surroundings, such as in thermal decomposition.
Reversible reactions are endothermic in one direction and exothermic in the other.
Enthalpy is a thermodynamic property linked to internal energy, represented by a capital H. We usually measure a change in enthalpy, represented by ∆H.
∆H = enthalpy of the products (H1) - enthalpy of the reactants (H2). We cannot measure enthalpy directly.
In exothermic reactions, ∆H is negative whereas in endothermic reactions, ∆H is positive.
∆H is always measured under standard conditions of 298K and 100kPa.
In reversible reactions, the ∆H value is the same numerical value forwards and backwards but the sign is reversed.
Reaction profiles are diagrams of enthalpy levels of reactants and products in a chemical reaction. They
In an endothermic reaction, product lines are higher enthalpy values than reactants. In an exothermic reaction, product lines are lower enthalpy values than reactants.
The difference between product and reactant lines is labelled as ∆H.
Values are measured in kJ mol-1.
Reaction pathways are shown with lines from the reactants to the products on enthalpy level diagrams. They plot enthalpy against reaction progress.
Reactions require an input of energy to break bonds before new bonds can form the products. The activation energy is the peak of the pathway above the enthalpy of reactants. It is the minimum amount of energy that reactants must have to react.
Standard enthalpy values are the ∆H values for enthalpy changes of specific reactions measured under standard conditions, represented by ⊖.
Standard enthalpy of reaction ( ΔHr⊖ ) is the enthalpy change when substances react under standard conditions in quantities given by the equation for the reaction.
Standard enthalpy of formation ( ΔfH⊖ ) is the enthalpy change when 1 mole of a compound is formed from its constitutent elements with all reactants and products in standard states under standard conditions.
The enthalpy of formation for an element is zero is it is in it’s standard state.
Standard enthalpy of combustion ( ΔcH⊖ ) is the enthalpy change when 1 mole of a substance is burned completely in excess oxygen with all reactants and products in their standard states under standard conditions.
Values for standard enthalpy of formation and combustion must be kept to per mole of what they refer.
Happy studying!
More Posts from Amateurchemstudent and Others

Plenty of opportunities to wear sunglasses this week! 😎 Here’s the science behind how the protect your eyes from the sun’s UV radiation in C&EN: https://ift.tt/2XW7h8L https://ift.tt/3gT8PI6
Combusting Alkanes
If you follow this blog, by now you must be thinking, when will we be done with the alkane chemistry? Well, the answer is never. There is still one more topic to touch on - burning alkanes and the environmental effects. Study up chums!
Alkanes are used as fuels due to how they can combust easily to release large amounts of heat energy. Combustion is essentially burning something in the presence of oxygen. There are two types of combustion: complete and incomplete.
Complete combustion occurs when there is a plentiful supply of air. When an alkane is burned in sufficient oxygen, it produces carbon dioxide and water. How much depends on what is being burnt. For example:
butane + oxygen -> carbon dioxide + water
2C4H10 (g) + 13O2 (g) -> 8CO2 (g) + 10H2O (g)
Remember state symbols in combustion reactions. In addition, this reaction can be halved to balance for 1 mole of butane by using fractions when dealing with the numbers.
C4H10 (g) + 6 ½ O2 (g) -> 4CO2 (g) + 5H2O (g)
Incomplete combustion on the other hand occurs when there is a limited supply of air. There are two kinds of incomplete combustion. The first type produces water and carbon monoxide.
butane + limited oxygen -> carbon monoxide + water
C4H10 (g) + 4 ½ O2 (g) -> 4CO (g) + 5H2O (g)
Carbon monoxide is dangerous because it is toxic and undetectable due to being smell-free and colourless. It reacts with haemoglobin in your blood to reduce their oxygen-carrying ability and can cause drowsiness, nausea, respiratory failure or death. Applicances therefore must be maintained to prevent the formation of the monoxide.
The other kind of incomplete combustion occurs in even less oxygen. It produces water and soot (carbon).
butane + very limited oxygen -> carbon + water
C4H10 (g) + 2 ½ O2 (g) -> 4C (g) + 5H2O (g)
Internal combustion engines work by changing chemical energy to kinetic energy, fuelled by the combustion of alkane fuels in oxygen. When this reaction is undergone, so do other unwanted side reactions due to the high pressure and temperature, e.g. the production of nitrogen oxides.
Nitrogen is regularly unreactive but when combined with oxygen, it produces NO and NO2 molecules:
nitrogen + oxygen -> nitrogen (II) oxide
N2 (g) + O2 (g) -> 2NO (g)
and
nitrogen + oxygen -> nitrogen (II) oxide
N2 (g) + 2O2 (g) -> 2NO2 (g)
Sulfur dioxide (SO2) is sometimes present in the exhaust mixture as impurities from crude oil. It is produced when sulfur reacts with oxygen. Nitrogen oxides, carbon dioxide, carbon monoxide, carbon particles, unburnt hydrocarbons, water vapour and sulfur dioxide are all produced in exhaust fumes and are also pollutants that cause problems you need to be aware of for the exam as well as how to get rid of them.
Greenhouse gases contribute to global warming, an important process where infrared radiation from the sun is prevented from escaping back into space by atmospheric gases. On the one hand, some greenhouse gases need to continue this so that the earth can sustain life as it traps heat, however, we do not want the earth’s temperature to increase that much. Global warming is the term given to the increasing average temperature of the earth, which has seen an increase in the last few years due to human activity - burning fossil fuels like alkanes has produced more gases which trap more heat. Examples of greenhouse gases include carbon dioxide, methane and water vapour.

Another pollution problem the earth faces is acid rain. Rain water is already slightly acidic due to the CO2 present in the atmosphere but acid rain is more acidic than this. Nitrogen oxides contribute to acid rain although sulfur dioxide is the main cause. The equation for sulfur dioxide reacting with water in the air to produce oxidised sulfurous acid and therefore sulphuric acid is:
SO2 (g) + H2O (g) + ½ O2 (g) -> H2SO4 (aq)
Acid rain is a problem because it destroys lakes, buildings and vegetation. It is also a global problem because it can fall far from the original source of the pollution.
Photochemical smog is formed from nitrogen oxides, sulfur dioxide and unburnt hydrocarbons that react with sunlight. It mostly forms in industralised cities and causes health problems such as emphysema.
So what can we do about the pollutants?
A good method of stopping pollution is preventing it in the first place, therefore cars have catalytic converters which reduce the amount of carbon monoxide, nitrogen oxides and unburnt hydrocarbons come into the atmosphere by converting them into less toxic gases. Shaped like a honeycomb for increased SA and therefore rate of conversion, platinum and rhodium coat ceramic and act as catalysts for the reactions that take place in an internal combustion engine.
As they pass over the catalyst, they react with each other to form less pollution:
octane + nitrogen (II) oxide -> carbon dioxide + nitrogen + water
C8H18 (g) + 25NO -> 8CO2 (g) + 12 ½ N2 (g) + 9H2O (g)
nitrogen (II) oxide + carbon monoxide -> carbon dioxide + nitrogen
2NO (g) + 2CO (g) -> 2CO2 (g) + N2 (g)
Finally, sulfur dioxide must be dealt with. The first way it is dealt with is by removing it from petrol before it can be burnt, however, this is often not economically favourable for fuels used in power stations. A process called flue gas desulfurisation is used instead.
In this, gases are passed through a wet semi-solid called a slurry that contains calcium oxide or calcium carbonate. These neutralise the acid, due to being bases, to form calcium sulfate which has little commercial value but can be oxidised to produce a more valuable construction material.
calcium oxide + sulfur dioxide -> calcium sulfite
CaO (s) + SO2 (g) -> CaSO3 (s)
calcium carbonate + sulfur dioxide -> calcium sulfite + carbon dioxide
CaCO3 (s) + SO2 (g) -> CaSO3 (s) + CO2 (g)
calcium sulfite + oxygen -> calcium sulfate
CaSO3 (s) + O -> CaSO4 (s)
SUMMARY
Alkanes are used as fuels due to how they can combust easily to release large amounts of heat energy. Combustion is essentially burning something in the presence of oxygen.
Complete combustion occurs when there is a plentiful supply of air. When an alkane is burned in sufficient oxygen, it produces carbon dioxide and water
Remember state symbols in combustion reactions. In addition, reactions can be halved to balance for 1 mole of compounds by using fractions when dealing with the numbers.
Incomplete combustion occurs when there is a limited supply of air. There are two kinds of incomplete combustion.
The first type produces water and carbon monoxide.
Carbon monoxide is dangerous because it is toxic and undetectable due to being smell-free and colourless. It reacts with haemoglobin in your blood to reduce their oxygen-carrying ability and can cause drowsiness, nausea, respiratory failure or death.
The other kind of incomplete combustion occurs in even less oxygen. It produces water and soot (carbon).
Internal combustion engines work by changing chemical energy to kinetic energy, fuelled by the combustion of alkane fuels in oxygen. When this reaction is undergone, so do other unwanted side reactions due to the high pressure and temperature, e.g. the production of nitrogen oxides.
Nitrogen is regularly unreactive but when combined with oxygen, it produces NO and NO2 molecules:
Sulfur dioxide (SO2) is sometimes present in the exhaust mixture as impurities from crude oil. It is produced when sulfur reacts with oxygen.
Nitrogen oxides, carbon dioxide, carbon monoxide, carbon particles, unburnt hydrocarbons, water vapour and sulfur dioxide are all produced in exhaust fumes and are also pollutants that cause problems you need to be aware of for the exam as well as how to get rid of them.
Greenhouse gases contribute to global warming, an important process where infrared radiation from the sun is prevented from escaping back into space by atmospheric gases. Some greenhouse gases need to continue this so that the earth can sustain life as it traps heat, however, we do not want the earth’s temperature to increase that much. Global warming is the term given to the increasing average temperature of the earth, which has seen an increase in the last few years due to human activity - burning fossil fuels like alkanes has produced more gases which trap more heat.
Another pollution problem the earth faces is acid rain. Nitrogen oxides contribute to acid rain although sulfur dioxide is the main cause.
Acid rain is a problem because it destroys lakes, buildings and vegetation. It is also a global problem because it can fall far from the original source of the pollution.
Photochemical smog is formed from nitrogen oxides, sulfur dioxide and unburnt hydrocarbons that react with sunlight. It mostly forms in industralised cities and causes health problems such as emphysema.
A good method of stopping pollution is preventing it in the first place, therefore cars have catalytic converters which reduce the amount of carbon monoxide, nitrogen oxides and unburnt hydrocarbons come into the atmosphere by converting them into less toxic gases. Shaped like a honeycomb for increased SA and therefore rate of conversion, platinum and rhodium coat ceramic and act as catalysts for the reactions that take place in an internal combustion engine.
As they pass over the catalyst, they react with each other to form less pollution.
octane + nitrogen (II) oxide -> carbon dioxide + nitrogen + water
C8H18 (g) + 25NO -> 8CO2 (g) + 12 ½ N2 (g) + 9H2O (g)
nitrogen (II) oxide + carbon monoxide -> carbon dioxide + nitrogen
2NO (g) + 2CO (g) -> 2CO2 (g) + N2 (g)
Finally, sulfur dioxide must be dealt with. The first way it is dealt with is by removing it from petrol before it can be burnt, however, this is often not economically favourable for fuels used in power stations. A process called flue gas desulfurisation is used instead.
In this, gases are passed through a wet semi-solid called a slurry that contains calcium oxide or calcium carbonate. Since they are bases, these neutralise the acid to form calcium sulfate which has little commercial value but can be oxidised to produce a more valuable construction material.
Happy studying!
Covalent Bonds: Sharing Is Caring!
Welcome to my second out of three posts on bonding - ionic, covalent and metallic. This post also covers the coordinate/ dative bond which I can’t remember if I’ve covered before. Only one more of this series left! Find the others here.
Covalent bonding involves one or more shared pairs of electrons between two atoms. These can be found in simple molecular elements and compounds like CO2 , macromolecular structures like diamond and molecular ions such as ammonium. Covalent bonds mostly occur between non-metals but sometimes metals can form covalent bonds.
Single covalent bonds share just one pair of electrons. Double covalent bonds share two. Triple covalent bonds share three.
Each atom usually provides one electron – unpaired in the orbital – in the bond. The number of unpaired electrons in an atom usually shows how many bonds it can make but sometimes atoms promote electrons to fit in more. Covalent bonds are represented with lines between the atoms – double and triple bonds represented with two and three lines respectively.
Dot and cross diagrams show the arrangement of electrons in covalent bonds. They use dots and crosses to demonstrate that the electrons come from different places and often only the outer shell is shown.

The simple explanation as to how atoms form covalent bonds is that one unpaired electron in the orbital of one atom overlaps with one in another atom. Sometimes atoms promote electrons in the same energy level to form more covalent bonds. For example, if an atom wants to make three covalent bonds but has a full 3s2 shell and a 3p1 shell, it can promote one of its 3s2 electrons so that an electron from the other atoms can fill the 3s shell and pair with the new 3p2 shell.
Sometimes promotion does not occur and that means different compounds can be made such as PCl3 or PCl5.

A lone pair of electrons is a pair of electrons from the same energy sub-level uninvolved in bonding. Sometimes these can form something called a coordinate bond, which contains a shared pair of electrons where both come from one atom. The lone pair of electrons is “donated” into the empty orbital of another atom to form a coordinate bond.

This is an example of a coordinate (sometimes called dative) bond between ammonia and a H+ ion which has an empty orbital. The lone pair on the ammonia overlaps with this H+ ion and donates its electrons. Both electrons come from the ammonia’s lone pair so it is a coordinate bond. This is demonstrated with an arrow. The diagram is missing an overall charge of + on the ammonium ion it produces. Coordinate bonds act the same as covalent bonds.
Once you have your covalent bonds, you need to know about covalent substances and their properties. There are two types of covalent substance: simple covalent (molecular) and macromolecular (giant covalent).
Molecular simply means that the formula for the compound or element describes exactly how many atoms are in one molecule, e.g. H2O. Molecular covalent crystalline substances usually exist as single molecules such as iodine or oxygen. They are usually gases or liquids at room temperature but can be low melting point solids.
Solid molecular covalent solids are crystalline so can be called molecular covalent crystals. Iodine and ice are examples of these. Iodine (shown below) has a regular arrangement which makes it a crystalline substance and water, as ice, has a crystalline structure as well.

The properties of these crystals are that they have low melting points, are very brittle due to the lack of strong bonds holding them together and also do not conduct electricity since no ions are present.
The other kind of covalent substance you need to know is macromolecular. This includes giant covalent structures such as diamond or graphite, which are allotropes of carbon. Non-metallic elements and compounds usually form these crystalline structures with a regular arrangement of atoms.
Allotropes are different forms of the same element in the same physical state.
Diamond is the hardest naturally occurring substance on earth therefore is good for cutting glass and drilling and mining. It has a high melting point due to the many covalent bonds which require a lot of energy to break. Each carbon has four of these bonds joining it to four others in a tetrahedral arrangement with a bond angle of 109.5 degrees and it does not conduct electricity or heat because there are no ions free to move.
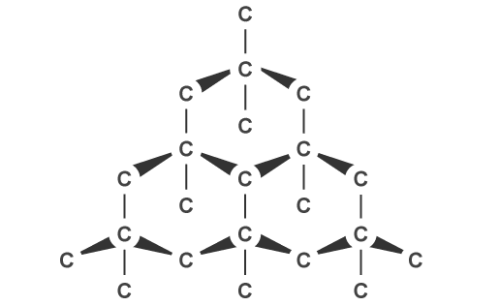
Graphite, on the other hand, can conduct electricity. This is because it has delocalised electrons between the layers which move and carry charge. Carbon atoms within the structure are only bonded to three others in a hexagonal arrangement with a bond angle of 120 degrees. Since only three of carbon’s unpaired electrons are used in bonding, the fourth becomes delocalised and moves between the layers of graphite causing weak attractions, explaining why it can conduct electricity.

Graphite’s layered structure and the weak forces of attractions between it make it a good lubricant and ideal for pencil lead because the layers can slide over each other. The attractions can be broken easily but the covalent bonds within the layers give graphite a high melting point due to the amount of energy needed to break them.
SUMMARY
Covalent bonding involves one or more shared pairs of electrons between two atoms. Covalent bonds mostly occur between non-metals but sometimes metals can form covalent bonds.
Single covalent bonds share just one pair of electrons. Double covalent bonds share two. Triple covalent bonds share three.
Each atom usually provides one electron – unpaired in the orbital – in the bond. The number of unpaired electrons in an atom usually shows how many bonds it can make but sometimes atoms promote electrons to fit in more. Covalent bonds are represented with lines between the atoms.
Dot and cross diagrams use dots and crosses to demonstrate that the electrons come from different places and often only the outer shell is shown.
The simple explanation as to how atoms form covalent bonds is that one unpaired electron in the orbital of one atom overlaps with one in another atom. Sometimes atoms promote electrons in the same energy level to form more covalent bonds.
Sometimes promotion does not occur and that means different compounds can be made such as PCl3 or PCl5.
A lone pair of electrons is a pair of electrons from the same energy sub-level uninvolved in bonding. Sometimes these can form something called a coordinate bond, which contains a shared pair of electrons where both come from one atom. The lone pair of electrons is “donated” into the empty orbital of another atom to form a coordinate bond.
The formation of ammonium is an example of this.
There are two types of covalent substance: simple covalent (molecular) and macromolecular (giant covalent).
Molecular simply means that the formula for the compound or element describes exactly how many atoms are in one molecule, e.g. H2O. Molecular covalent crystalline substances usually exist as single molecules such as iodine or oxygen. They are usually gases or liquids at room temperature but can be low melting point solids.
Solid molecular covalent solids are crystalline so can be called molecular covalent crystals. Iodine and ice are examples of these.
The properties of these crystals are that they have low melting points, are very brittle due to the lack of strong bonds holding them together and also do not conduct electricity since no ions are present.
Giant covalent structures such as diamond or graphite are allotropes of carbon. Allotropes are different forms of the same element in the same physical state.
Diamond has a high melting point due to the many covalent bonds which require a lot of energy to break. Each carbon has four of these bonds joining it to four others in a tetrahedral arrangement with a bond angle of 109.5 degrees and it does not conduct electricity or heat because there are no ions free to move.
Graphite can conduct electricity. This is because it has delocalised electrons between the layers which move and carry charge. Carbon atoms within the structure are only bonded to three others in a hexagonal arrangement with a bond angle of 120 degrees. Since only three of carbon’s unpaired electrons are used in bonding, the fourth becomes delocalised and moves between the layers of graphite causing weak attractions, explaining why it can conduct electricity.
Graphite’s layered structure and the weak forces of attractions between it make it a good lubricant and ideal for pencil lead because the layers can slide over each other. The attractions can be broken easily but the covalent bonds within the layers give graphite a high melting point due to the amount of energy needed to break them.
Happy studying!
Biochemistry
Update: Pictures are working!
Atoms
There are a few basic chemistry concepts that are essential to understand. For starters, understanding what an atom is and its basic properties.
Atoms are the building block of all matter. They have a positive nucleus, with positive protons, and neutral neutrons. In a large area surrounding the nucleus, is the electron cloud, made of negatively charged electrons.
An atom in its elemental state is always neutral.
When an element has a charge, it is because it has an unequal number of protons an electrons, making it an ion. Sometimes an element’s nucleus has an unequal number of neutrons and protons, making it an isotope. Carbon-14, for example, has 8 neutrons, instead of the 6 that Carbon-12 has. Carbon-14 is also a radioisotope, meaning it emits particles and decays at a rate called a half-life, making it useful for fossil dating. Along with that, radioactive carbon can be used as a tracer. This means it is incorporated in CO2 molecules and used to track metabolic pathways.
The location of the electron affects how the atom will react with other elements. When electrons are in the lowest available energy level, they are in the ground state. When they absorb energy, they move to a higher energy level, entering the excited state. For instance, when chlorophyll absorbs light energy, electrons within it are boosted to higher energy levels. This provides the energy necessary to produce sugar when they return to their ground state level as they release the energy they absorbed.
Bonding
Elements bond when two nuclei are attracted to each other. Energy is released when a bond is formed. All atoms want to either get rid of all their electrons on their outer shell or fill their outer shell with 8 (or in hydrogen’s case, 2) electrons, which makes them stable. There are 3 kinds of bonds, but for biochemistry, Ionic and covalent bonds are what is relevant.
Ionic bonds form ions (hence the name.) They occur when electrons are transferred. The atom that gains electrons becomes a negatively charged anion. The atom that loses electrons becomes a positively charged cation.
Covalent bonds are made when electrons are shared. This occurs when the two atoms have electronegativities that are closer together than in an ionic bond. Electronegativity is the tendency of an atom to pull electrons towards it. These bonds can be polar if the electronegativity is high enough. A polar molecule is a molecule with a partial charge. For example, water is a polar molecule, as oxygen is extremely electronegative, and water is partially electronegative.

Hydrogen Bonding
Hydrogen bonding is a specific kind of intermolecular force that is essential to life. It is what keeps the 2 strands of DNA bonded together, and gives water its unique characteristics. Since oxygen has a partial negative charge, and hydrogen has a partial positive charge, they are naturally drawn to each other.

Hydrophobic vs Hydrophilic
Polar molecules are hydrophilic. This is because they are attracted to the partially charged ends of water. Hydrophilic means they are attracted to water. (Not in that way… sick) NaCl or table salt is hydrophilic. This is why salt dissolves in water.
Non-polar molecules are hydrophobic. This means they are repelled by water. (They’re filthy water haters.) Lipids are hydrophobic, which is why fats and oils do not dissolve in water.
The cell membrane is a phospholipid bilayer, only allowing nonpolar substances to dissolve through it. Large polar molecules have to use specific hydrophilic channels.
Characteristics of Water
Water is a unique molecule, and without its unique properties, life on earth would not exist as it does, or even at all.
Water has a high specific heat: Because hydrogen bonds are so strong, it requires a lot of heat energy to break them. This is why large bodies of water remain the same temperature, and why coastal cities have a consistent temperature because the water absorbs all the heat energy before it can warm up.
Water has a high heat of vaporisation: A large amount of energy is needed for water to vaporise, which is why sweating is such an effective cooling method.
Water has high adhesion properties: Adhesion is when one substance clings to another. Adhesion causes capillary action, which occurs in the xylem of plants, and is used to bring water up from the roots without expending energy.
Water is a universal solvent: Due to its high polarity, water makes an excellent solvent.
Water is extremely cohesive: Molecules of water tend to stick to each other. This is observed in surface tension and allows for small insects to run across the surface of the water. Cohesion is also necessary to bring water up from the roots, by transpirational-pull cohesion tension.
Ice is less dense than water: Instead of freezing all the way through, ice crystallises, leaving large amounts of space, causing ice to float. This is essential for the survival of marine life during the winter, as they can live beneath the ice.
pH
pH is calculated by taking the -log of the chance of finding hydronium (H30+) ions within a certain amount of water. Hydronium is made in rare circumstances, where a hydrogen ion breaks off from a water molecule. Normally, there is a 1 in 10 million chance of there being a hydronium ion. This is the equivalent of 1x10^-7. The -log of this number is 7, the neutral pH.
Any pH below 7 is acidic. Any pH above 7 is basic. Stomach acid has a pH of 2, while bleach has a pH of 11. Human blood has a pH of around 7.4
Most living cells need to have an internal environment with a pH of around 7. Buffers exist to regulate pH by either absorbing excess hydrogen ions or donating missing hydrogen ions. In human blood, the bicarbonate ion (HCO3) is essential.
Macromolecules
There are 4 types of macromolecules: carbohydrates, lipids, proteins, and nucleic acids.
Carbohydrates
Carbohydrates are made of carbon, hydrogen, and oxygen. They supply quick and easy energy. 1 gram of all carbohydrates will release 4 calories of energy. In our diet, they can be found almost everywhere in foods such as rice, pasta, bread, cookies, etc.
There are 3 kinds of carbohydrates: monosaccharides, disaccharides, and polysaccharides.
Monosaccharides
All monosaccharides have a chemical formula of C6H12O6. It is the placement of the carbon, oxygen, and hydrogen that determines its properties. Glucose, fructose, and galactose are all examples. They are isomers, meaning they have the same chemical formula, but a different structure.

Disaccharides
When 2 monosaccharides join together, they create disaccharides. They all have the chemical formula C12H22O11. Dehydration synthesis is the process that creates them. This process releases 1 molecule of water, hence the name. Lactose, maltose, and sucrose are all examples.
Hydrolysis is the exact opposite of dehydration synthesis. It is used during digestion. One molecule of water is used to breakdown polymers into monomers.
Polysaccharides Polysaccharides are long polymers of carbohydrates. Cellulose (plant cell wall), chitin (exoskeleton, fungi cell wall), glycogen (how animals store carbohydrates) and starch (how plants store carbohydrates) are all examples.
Lipids
Lipids include fats, oils, and waxes. Most contain 1 glycerol and 3 fatty acids. Glycerol is alcohol.

Fatty acids are the building blocks of lipids and are hydrocarbon chains with carboxyl groups at the end. There are 2 varieties; saturated and unsaturated. (3 if you count trans-fats when extra hydrogen is added to the fat to make the lipid solid)
Saturated fats are solid at room temperature, and are famously unhealthy as they are linked to heart disease.
Unsaturated fats are liquid at room temperature and are good dietary fats.

Lipids store much more energy than carbohydrates. 1 gram of any lipid will release 9 calories of heat per gram. They can be structural, as in the phospholipids of the cell membrane, or they can be hormones.
Proteins
Proteins are polymers of amino acids linked together by peptide bonds.
Amino acids are identifiable by their carboxyl group, amine group, and variable R, attached to a central carbon atom.
Proteins are complex and perform a vast array of duties, such as growth and repair, being enzymes, membrane channels, and hormones.
1 gram of protein releases 4 calories of heat.
Proteins contain the elements C H O N P S
There are only 20 amino acids coding for the thousands of proteins in the human body.
Protein Structure
There are 4 levels to the structure of a protein.
The primary structure results from the sequence of amino acids making up the polypeptide
The secondary structure results from hydrogen bonding within the molecule. This causes a helical structure
The tertiary structure is an intricate 3-dimensional shape or conformation of a protein and most directly decides the function of the protein. Enzymes denature in high temperatures or in the wrong pH because the tertiary structure is compromised.
The quaternary structure is only found in proteins that have more than 1 polypeptide chain, such as in haemoglobin.

Enzymes
Enzymes are large proteins
Enzymes lower the energy of activation, speeding up the reaction, as it lowers the amount of energy needed to start the reaction.
The chemical an enzyme works on is known as a substrate
Enzymes are specifically designed for specific substrates. For example, lactase only works on lactose. Notice the naming pattern for enzymes and their substrates.
The induced fit model is an explanation for how they work. When the substrate enters the active site, it induces the enzyme to change its shape to fit the substrate.
Enzymes can be reused as they do not degrade during a reaction
Enzymes are assisted by cofactors (minerals) or coenzymes (vitamins)



Prions
Prions are proteins that cause diseases. Mad cow disease is an example. It is a misformed protein able to influence other proteins to fold in the same way.
Nucleic Acids
There are 2 kinds of nucleic acids: RNA and DNA. They are necessary for carrying genetic information.
Nucleic acids are polymers of nucleotides
The nucleotides are the two purines: Adenine and Guanine, and the 3 pyrimidines, Thymine, Uracil, and Cytosine. Uracil is only found in RNA, and thymine is only found in DNA. Adenine connects with thymine/uracil, and guanine connects with cytosine.


#OTD a year ago, Moderna’s RNA vaccine became the first #COVID19 vaccine to enter phase 1 trials. The latest #ChemVsCOVID graphic with the Royal Society of Chemistry takes a brief look at how prior research helped COVID vaccines reach this point quickly: https://ift.tt/3cE5xHR https://ift.tt/3rV4v0F
It’s day 5 of #ChemAdvent – here’s why peppermint candy canes make your mouth feel cold! bit.ly/chemadvent2020 https://ift.tt/2JM6bZ7
-
 amateurchemstudent reblogged this · 4 years ago
amateurchemstudent reblogged this · 4 years ago -
 amateurchemstudent liked this · 4 years ago
amateurchemstudent liked this · 4 years ago -
 zoetax liked this · 5 years ago
zoetax liked this · 5 years ago -
 khadooy8 reblogged this · 5 years ago
khadooy8 reblogged this · 5 years ago -
 jade12358 liked this · 6 years ago
jade12358 liked this · 6 years ago -
 dolce-jasmine-blog liked this · 6 years ago
dolce-jasmine-blog liked this · 6 years ago -
 bygollyyoureright liked this · 6 years ago
bygollyyoureright liked this · 6 years ago -
 electronicstarlightfestival liked this · 7 years ago
electronicstarlightfestival liked this · 7 years ago -
 princessx-grunge reblogged this · 7 years ago
princessx-grunge reblogged this · 7 years ago -
 poorarthoes reblogged this · 7 years ago
poorarthoes reblogged this · 7 years ago -
 emsstudy-blog liked this · 7 years ago
emsstudy-blog liked this · 7 years ago -
 blueberry-pancakes-stuff liked this · 7 years ago
blueberry-pancakes-stuff liked this · 7 years ago -
 r-elisetheconstantchaos liked this · 7 years ago
r-elisetheconstantchaos liked this · 7 years ago -
 as-studypeach reblogged this · 7 years ago
as-studypeach reblogged this · 7 years ago -
 malfoyfever-blog reblogged this · 7 years ago
malfoyfever-blog reblogged this · 7 years ago -
 malfoyfever-blog liked this · 7 years ago
malfoyfever-blog liked this · 7 years ago -
 quantumskies-blog liked this · 7 years ago
quantumskies-blog liked this · 7 years ago -
 bulletjoinspo reblogged this · 7 years ago
bulletjoinspo reblogged this · 7 years ago -
 pplanet-curiosityy liked this · 7 years ago
pplanet-curiosityy liked this · 7 years ago -
 gsmtz-blog liked this · 7 years ago
gsmtz-blog liked this · 7 years ago -
 as-studypeach reblogged this · 7 years ago
as-studypeach reblogged this · 7 years ago



